Label: LIDOCAINE- lidocaine hydrochloride injection, solution
- NDC Code(s): 63323-208-01, 63323-208-05
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 20, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONINTRAVENOUS FOR CARDIAC ARRHYTHMIAS
-
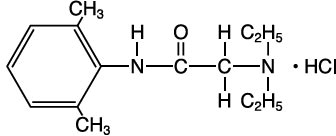
DESCRIPTIONLidocaine hydrochloride, chemical name: acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride has the following structural formula: Lidocaine ...
-
CLINICAL PHARMACOLOGYLidocaine hydrochloride exerts its antiarrhythmic effect by raising the electrical stimulation threshold of the ventricle during diastole. In usual therapeutic doses, lidocaine hydrochloride ...
-
INDICATIONS AND USAGELidocaine Hydrochloride Injection, USP administered intravenously is specifically indicated in the acute management of 1) ventricular arrhythmias occurring during cardiac manipulation such as ...
-
CONTRAINDICATIONSLidocaine Hydrochloride Injection, USP is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type. Lidocaine Hydrochloride Injection, USP should ...
-
WARNINGSConstant monitoring of the electrocardiogram and blood pressure is essential in the proper administration of Lidocaine Hydrochloride Injection, USP intravenously. If hypotension or excessive ...
-
PRECAUTIONSThe safe use of Lidocaine Hydrochloride Injection, USP requires careful ECG observation in an environment equipped and by persons trained for resuscitation. General - Caution should be employed ...
-
ADVERSE REACTIONSTo report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Most adverse reactions accompanying administration of ...
-
OVERDOSAGEManagement of Adverse Reactions— 1. In the case of severe reaction, discontinue the use of Lidocaine Hydrochloride Injection, USP. 2. Institute emergency resuscitative procedures and administer ...
-
DOSAGE AND ADMINISTRATIONAdult - For Direct Injection–The usual dose is 50 to 100 mg administered intravenously under ECG monitoring. This dose may be administered at the rate of approximately 25 to 50 mg/min ...
-
HOW SUPPLIED:For Direct Injection Only - Lidocaine Hydrochloride Injection, USP - Product - Code - Unit of Sale - Strength - Each - 20805 - NDC 63323-208-05 - Unit of 25 - 2% 100 mg per 5 mL - (20 ...
-
SPL UNCLASSIFIED SECTIONwww.fresenius-kabi.com/us - 45775D - Revised: May 2022
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Lidocaine HCl 5 mL Single Dose Vial Label - NDC 63323-208-01 20805 - LIDOCAINE HCl - INJECTION, USP - 2% 100 mg per 5 mL - (20 mg per ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Lidocaine HCl 5 mL Vial Tray Label - NDC 63323-208-05 20805 - LIDOCAINE HCl - INJECTION, USP - 2% 100 mg per 5 mL - (20 mg per mL) Intravenous For ...
-
INGREDIENTS AND APPEARANCEProduct Information