NOT FOR INJECTION. Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of steroid medication in ...
NOT FOR INJECTION. Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of steroid medication in the treatment of herpes simplex requires great caution; frequent slit lamp microscopy is recommended.
Prolonged use may result in glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and posterior subcapsular cataract formation. Prolonged use may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions or parasitic infections of the eye, steroids may mask infection or enhance existing infection.
In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids.
If this product is used for 10 days or longer, intraocular pressure (IOP) should be routinely monitored even though it may be difficult in children and uncooperative patients. Steroids should be used with caution in the presence of glaucoma. IOP should be checked frequently.
The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation.
Products containing neomycin sulfate may cause cutaneous sensitization. Sensitivity to topically administered aminoglycosides, such as neomycin, may occur in some patients. Severity of hypersensitivity reactions may vary from local effects to generalized reactions such as erythema, itching, urticaria, skin rash, anaphylaxis, anaphylactoid reactions, or bullous reactions. If hypersensitivity develops during use of the product, treatment should be discontinued. Cross-hypersensitivity to other aminoglycosides can occur, and the possibility that patients who become sensitized to topical neomycin may also be sensitive to other topical and/or systemic aminoglycosides should be considered.
General
The initial prescription and renewal of the medication order beyond 8 g of Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Ointment should be made by a physician only after examination of the patient with the aid of magnification, such as a slit lamp biomicroscopy and, where appropriate, fluorescein staining.
The possibility of persistent fungal infections of the cornea should be considered after prolonged steroid dosing. Fungal infection should be suspected in patients with persistent corneal ulceration.
Information for Patients
If inflammation or pain persists longer than 48 hours or becomes aggravated, the patient should be advised to discontinue use of the medication and consult a physician.
This product is sterile when packaged. To prevent contamination, care should be taken to avoid touching the tube tip to eyelids or to any other surface. The use of this tube by more than one person may spread infection. Keep tube tightly closed when not in use. Keep out of reach of children.
Patients should be advised that their vision may be temporarily blurred following dosing with Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Ointment. Care should be exercised in operating machinery or driving a motor vehicle.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic or mutagenic potential have not been conducted with polymyxin B sulfate. Treatment of cultured human lymphocytes in vitro with neomycin increased the frequency of chromosome aberrations at the highest concentration (80 μg/mL) tested. However, the effects of neomycin on carcinogenesis and mutagenesis in humans are unknown. Polymyxin B has been reported to impair the motility of equine sperm, but its effects on male or female fertility are unknown.
Pregnancy
Dexamethasone has been shown to be teratogenic in mice and rabbits following topical ophthalmic application in multiples of the therapeutic dose.
In the mouse, corticosteroids produce fetal resorptions and a specific abnormality, cleft palate. In the rabbit, corticosteroids have produced fetal resorptions and multiple abnormalities involving the head, ears, limbs, palate, etc.
There are no adequate or well-controlled studies in pregnant women. However, prolonged or repeated corticoid use during pregnancy has been associated with an increased risk of intra-uterine growth retardation. Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Ointment should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the embryo or fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy should be observed carefully for signs of hypoadrenalism.
Nursing Mothers
Systemically administered corticosteroids appear in human milk, could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Ointment is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
No overall clinical differences in safety or effectiveness have been observed between the elderly and other adult patients.
Close



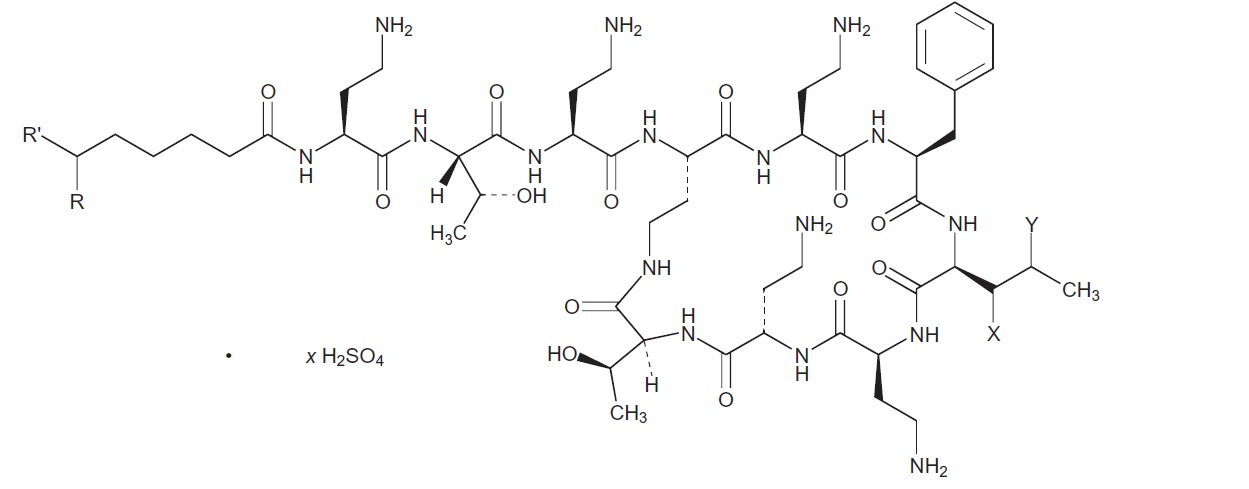
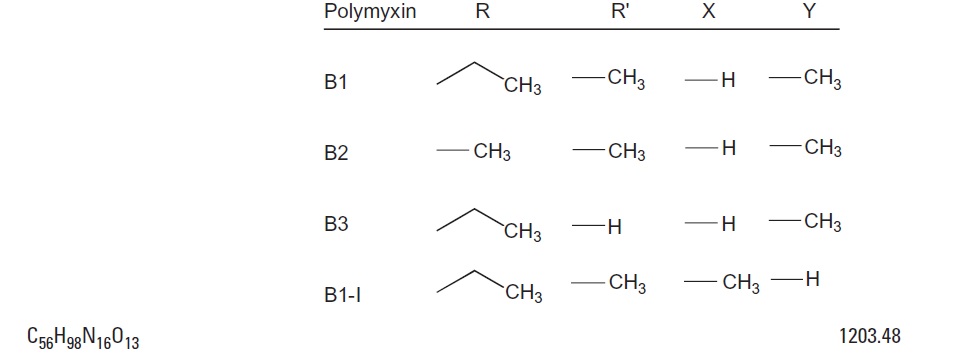
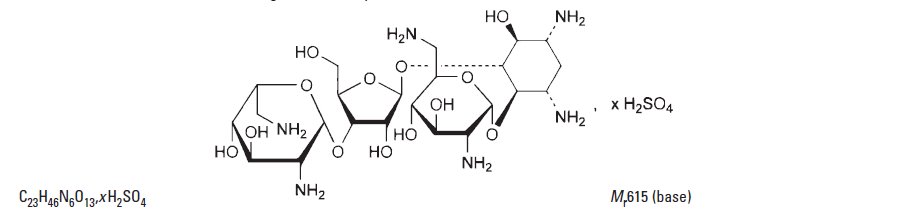 The chemical structure for the active ingredient Polymyxin B Sulfate is:
The chemical structure for the active ingredient Polymyxin B Sulfate is:
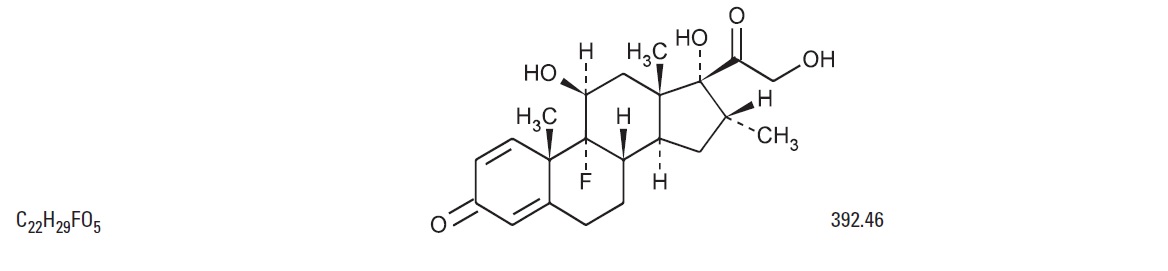
 The chemical structure for the active ingredient Dexamethasone is:
The chemical structure for the active ingredient Dexamethasone is: