Label: MEDI FIRST SINUS PAIN AND PRESSURE- acetaminophen, phenylephrine hydrochloride tablet
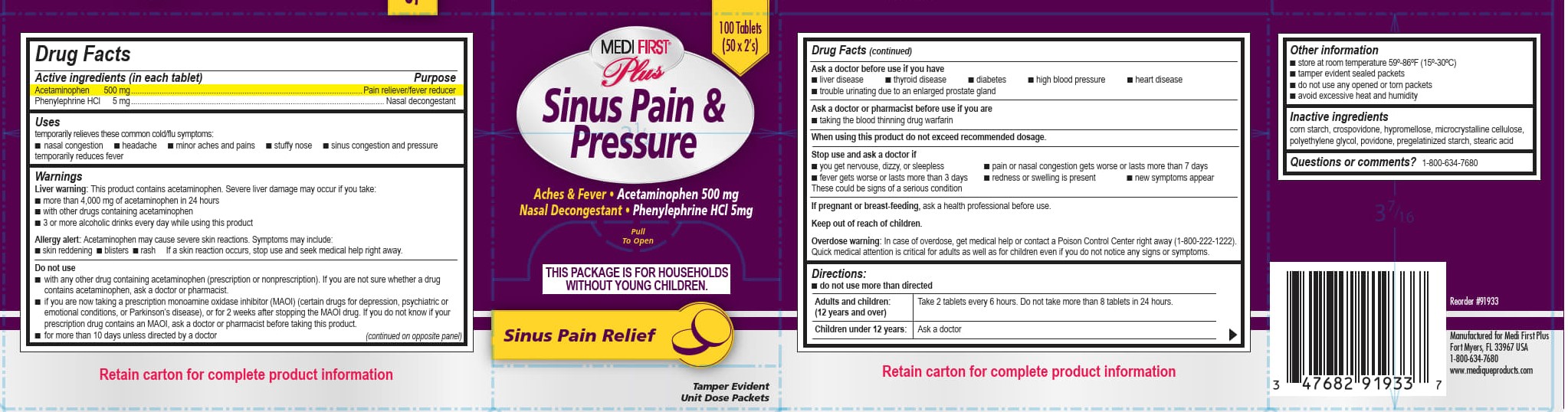
MEDI FIRST PLUS SINUS PAIN AND PRESSURE- acetaminophen, phenylephrine hydrochloride tablet
OTIS CLAPP MYGREX- acetaminophen, phenylephrine hydrochloride tablet
-
NDC Code(s):
47682-509-03,
47682-509-99,
47682-819-13,
47682-819-33, view more47682-819-48, 47682-819-50, 47682-819-99, 47682-919-33, 47682-919-48
- Packager: Unifirst First Aid Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
■ more than 4,000 mg of acetaminophen in 24 hours
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
■ skin reddening
■ blisters
■ rash
If a skin reaction occurs, stop use and seek medical help right away.
-
DO NOT USE
Do not use
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
■ for more than 10 days unless directed by a doctor
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- Medi-First Plus Sinus Pain and Pressure Label
-
Otis Clapp Mygrex Label
Otis Clapp
Quality and Integrity Since 1840
MYGREX ™
Pain Reliever-Decongestant
Advanced Relief
For Sinus/Headaches
See Warnings and Directions on Side PanelThis package is for Households without Young Children.
Acetaminophen 500 mg • Aches & Fever
Phenylephrine HCl 5 mg • Nasal Decongestant
Tear Out Along Perforation To Dispense
PROFESSIONAL HEALTHCARE
300 Tablets (150 PACKETS OF 2)
- Medi-First Sinus Pain and Pressure Label
-
INGREDIENTS AND APPEARANCE
MEDI FIRST SINUS PAIN AND PRESSURE
acetaminophen, phenylephrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47682-819 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 68401960MK) POVIDONE (UNII: FZ989GH94E) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 12mm Flavor Imprint Code AZ;261 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-819-33 50 in 1 BOX 12/30/2008 1 2 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:47682-819-48 125 in 1 BOX 12/30/2008 2 2 in 1 PACKET; Type 0: Not a Combination Product 3 NDC:47682-819-13 250 in 1 BOX 12/30/2008 3 NDC:47682-819-99 2 in 1 PACKET; Type 0: Not a Combination Product 4 NDC:47682-819-99 2 in 1 PACKET; Type 0: Not a Combination Product 12/30/2008 5 NDC:47682-819-50 25 in 1 BOX 04/16/2019 5 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/30/2008 MEDI FIRST PLUS SINUS PAIN AND PRESSURE
acetaminophen, phenylephrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47682-919 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 68401960MK) POVIDONE (UNII: FZ989GH94E) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 12mm Flavor Imprint Code AZ;261 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-919-33 50 in 1 BOX 12/30/2008 1 2 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:47682-919-48 125 in 1 BOX 12/30/2008 2 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/30/2008 OTIS CLAPP MYGREX
acetaminophen, phenylephrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47682-509 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 68401960MK) POVIDONE (UNII: FZ989GH94E) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 12mm Flavor Imprint Code AZ;261 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-509-03 150 in 1 BOX 12/30/2008 1 NDC:47682-509-99 2 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:47682-509-99 2 in 1 PACKET; Type 0: Not a Combination Product 12/30/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/30/2008 Labeler - Unifirst First Aid Corporation (832947092)