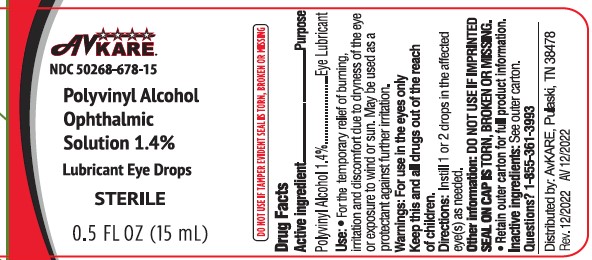
Label: POLYVINYL ALCOHOL solution/ drops
- NDC Code(s): 50268-678-15
- Packager: AvPAK
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
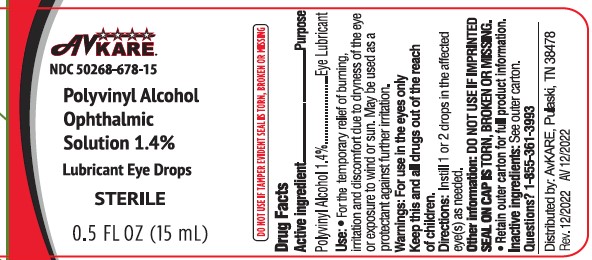
Active ingredientPolyvinyl Alcohol 1.4%
-
PurposeLubricant
-
Usesfor the temporary relief of burning, irritation and discomfort due to dryness of the eye or exposure to wind or sun - may be used as a protectant against further irritation
-
WarningsDo not use if solution changes color or becomes cloudy - When using this product - Avoid contamination, do not touch tip of container to any surface. Replace cap ...
-
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222)
-
DirectionsShake well before use - instill 1 to 2 drops in the affected eye(s) as needed
-
Other informationStore at room temperature 15-30C (59-86F) Do No Use if imprinted seal on cap is torn, broken or missing - Discard 90 days after opening - Retain outer carton for full product information
-
Inactive ingredientsdibasic sodium phosphate, edetate disodium, monobasic sodium phosphate, purified water, sodium chloride. Phosphoric acid and/or sodium hydroxide may be added to adjust pH. PRESERVATIVE ADDED ...
-
Questions ?Call 1-855-361-3993 - Distributed by: AvKARE - Pulaski, TN 38478 - www.avkare.com - Rev. 12/2022 AV 12/2022
-
Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCEProduct Information