Label: SULFAMETHOXAZOLE AND TRIMETHOPRIM suspension
- NDC Code(s): 60687-442-24, 60687-442-53, 60687-442-75
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 65862-496
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim oral suspension and other antibacterial drugs, sulfamethoxazole and ...
-
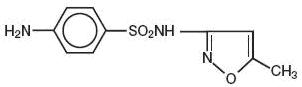
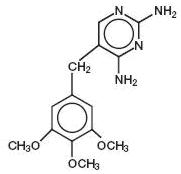
DESCRIPTIONSulfamethoxazole and trimethoprim oral suspension, USP is a synthetic antibacterial combination product containing 200 mg sulfamethoxazole and 40 mg trimethoprim in each teaspoonful (5 ...
-
CLINICAL PHARMACOLOGYSulfamethoxazole and trimethoprim is rapidly absorbed following oral administration. Both sulfamethoxazole and trimethoprim exist in the blood as unbound, protein-bound and metabolized forms ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim oral suspension and other antibacterial drugs, sulfamethoxazole and ...
-
CONTRAINDICATIONSSulfamethoxazole and trimethoprim oral suspension is contraindicated in the following situations: known hypersensitivity to trimethoprim or sulfonamides - history of drug-induced immune ...
-
WARNINGSEmbryofetal Toxicity - Some epidemiologic studies suggest that exposure to sulfamethoxazole and trimethoprim during pregnancy may be associated with an increased risk of congenital malformations ...
-
PRECAUTIONSDevelopment of Drug Resistant Bacteria - Prescribing sulfamethoxazole and trimethoprim oral suspension in the absence of a proven or strongly suspected bacterial infection or a prophylactic ...
-
ADVERSE REACTIONSThe following adverse reactions associated with the use of sulfamethoxazole and trimethoprim oral suspension or sulfamethoxazole and trimethoprim were identified in clinical trials, postmarketing ...
-
OVERDOSAGEAcute - The amount of a single dose of sulfamethoxazole and trimethoprim that is either associated with symptoms of overdosage or is likely to be life-threatening has not been reported. Signs ...
-
DOSAGE AND ADMINISTRATIONSulfamethoxazole and trimethoprim oral suspension is contraindicated in pediatric patients less than 2 months of age. Urinary Tract Infections and Shigellosis in Adults and Pediatric Patients ...
-
HOW SUPPLIEDSulfamethoxazole and Trimethoprim Oral Suspension, USP contains 200 mg sulfamethoxazole and 40 mg trimethoprim in each teaspoonful (5 mL). Available as a pink, cherry-flavored syrup suspension ...
-
REFERENCESKremers P, Duvivier J, Heusghem C. Pharmacokinetic Studies of Co-Trimoxazole in Man after Single and Repeated Doses. J Clin Pharmacol. Feb-Mar 1974; 14:112–117. Kaplan SA, et al ...
-
PACKAGING INFORMATIONAmerican Health Packaging unit dose cups (see - How Supplied section) contain drug product from Aurobindo Pharma USA, Inc. as follows: (800 mg/160 mg per 20 mL / 40 UD) NDC 60687-442-75 ...
-
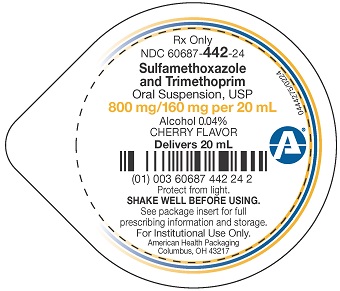
Package/Label Display PanelSulfamethoxazole and - Trimethoprim Oral - Suspension, USP - Rx Only - Contains alcohol 0.04% and added - as preservatives methylparaben 0.1%, sodium benzoate 0.1% CHERRY ...
-
Package/Label Display PanelRx Only - NDC 60687- 442-24 - Sulfamethoxazole - and Trimethoprim - Oral Suspension, USP - 800 mg/160 mg per 20 mL - Alcohol 0.04% CHERRY FLAVOR - Delivers 20 mL - Protect from ...
-
INGREDIENTS AND APPEARANCEProduct Information