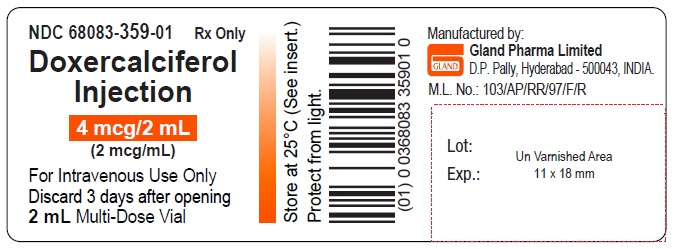
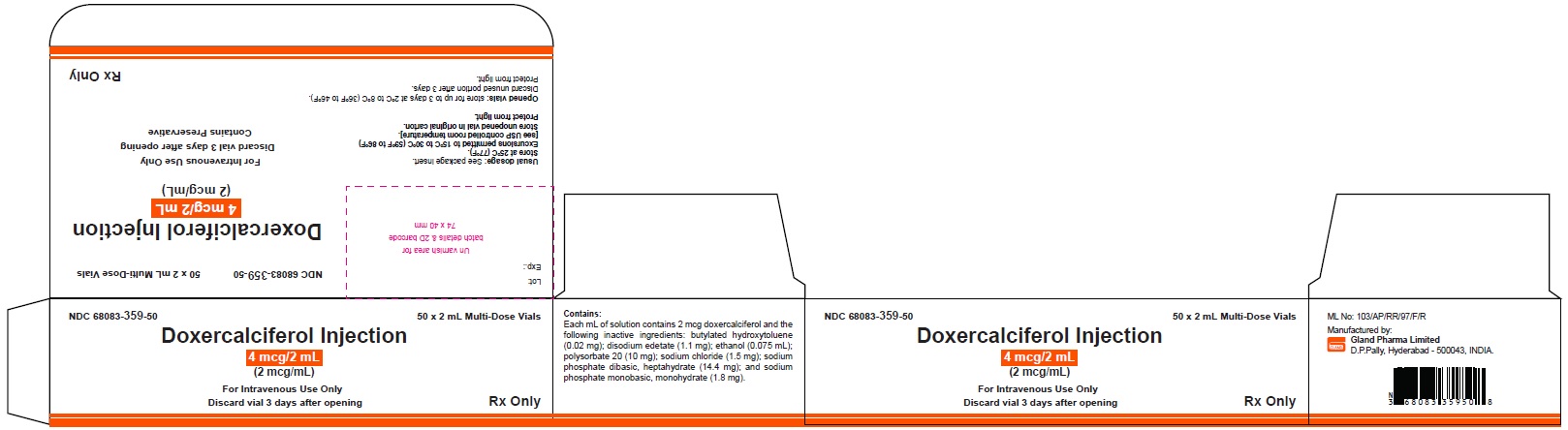
Label: DOXERCALCIFEROL injection, solution
- NDC Code(s): 68083-300-01, 68083-300-50, 68083-359-01, 68083-359-50
- Packager: Gland Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Doxercalciferol Injection safely and effectively. See full prescribing information for Doxercalciferol Injection. Doxercalciferol ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE• Doxercalciferol Injection is indicated for the treatment of secondary hyperparathyroidism in adult patients with CKD on dialysis.
-
2 DOSAGE AND ADMINISTRATION2.1 Prior to Initiation of Doxercalciferol Injection - • Ensure serum calcium is not above the upper limit of normal before initiating treatment with Doxercalciferol Injection [see Warnings and ...
-
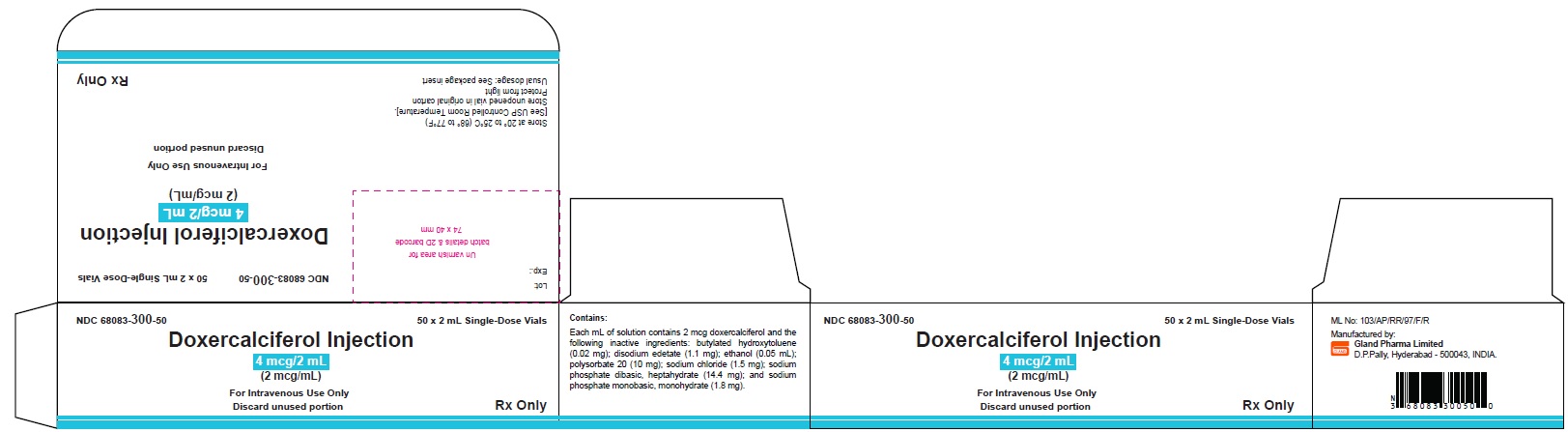
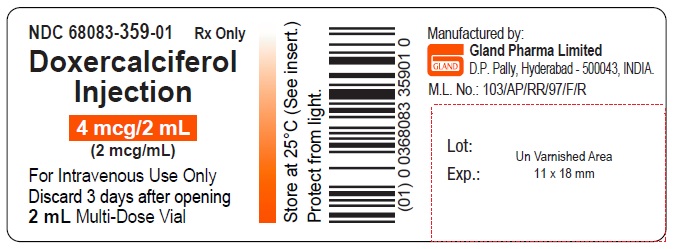
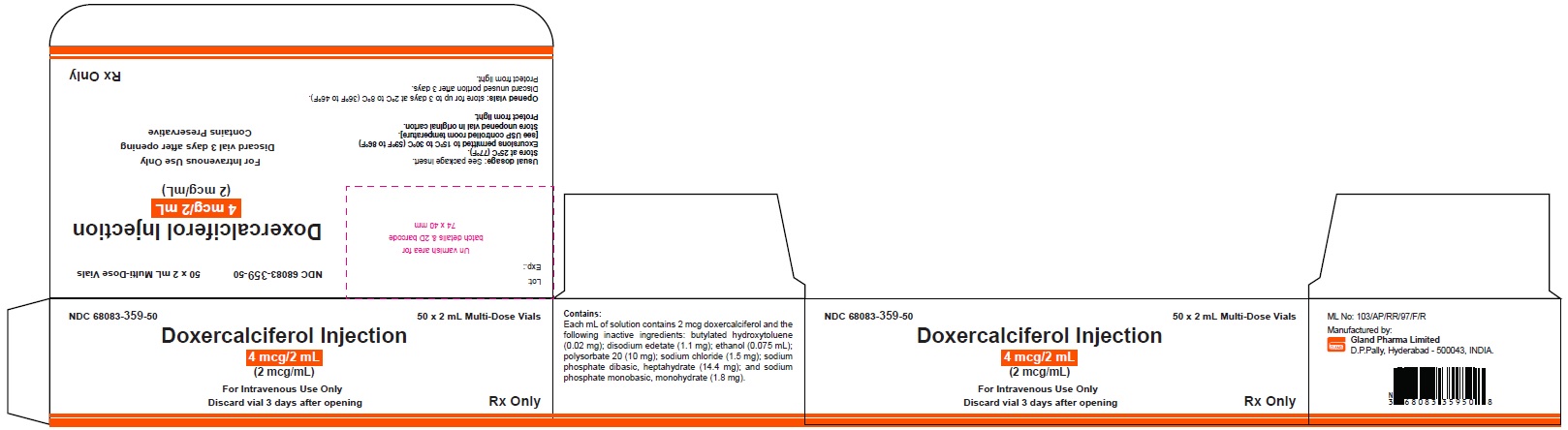
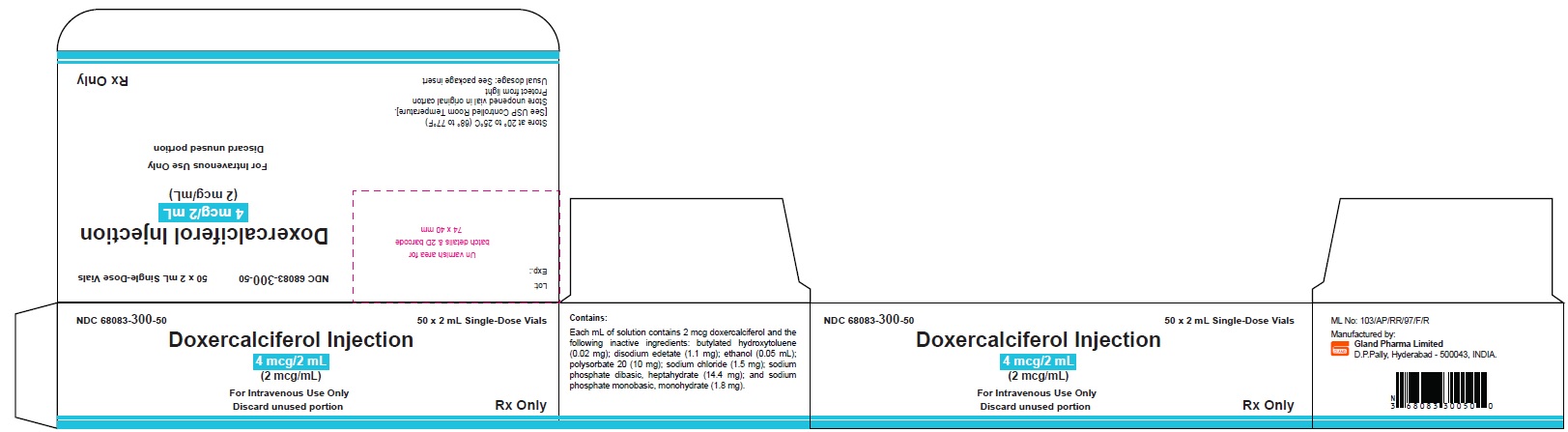
3 DOSAGE FORMS AND STRENGTHSInjection: clear and colorless solution available as follows: • 4 mcg/2 mL (2 mcg/mL) single-dose vial - • 4 mcg/2 mL (2 mcg/mL) multiple-dose vial
-
4 CONTRAINDICATIONSDoxercalciferol Injection is contraindicated in patients with: • Hypercalcemia [see Warnings and Precautions (5.1)] • Vitamin D toxicity [see Warnings and Precautions (5.1)] • Known ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypercalcemia - Hypercalcemia may occur during Doxercalciferol Injection treatment. Acute hypercalcemia may increase the risk of cardiac arrhythmias and seizures and may potentiate the ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in another section of the label: • Hypercalcemia [see Warnings and Precautions (5.1)] • Serious Hypersensitivity Reactions ...
-
7 DRUG INTERACTIONSTables 2 include clinically significant drug interactions with Doxercalciferol Injection. Table 2: Clinically Significant Drug Interactions with Doxercalciferol ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data with Doxercalciferol Injection in pregnant women are insufficient to identify a drug-associated risk for major birth defects ...
-
10 OVERDOSAGEOverdosage of Doxercalciferol Injection may lead to hypercalcemia, hypercalciuria, and hyperphosphatemia [see Warnings and Precautions (5.1)]. The treatment of acute overdosage should consist of ...
-
11 DESCRIPTIONDoxercalciferol Injection contains doxercalciferol, which is a synthetic vitamin D2 analog. Doxercalciferol undergoes metabolic activation in vivo to form 1α,25-dihydroxyvitamin D2 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Doxercalciferol is a synthetic vitamin D2 analog that requires metabolic activation to form the active 1α,25-(OH)2D2 metabolite, which binds to the vitamin D receptor ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 104-week carcinogenicity study in rats, there was an increased incidence of benign and malignant adrenal pheochromocytomas in ...
-
14 CLINICAL STUDIES14.3 Clinical Studies of Doxercalciferol Injection in Patients with CKD on Dialysis - The safety and effectiveness of Doxercalciferol Injection were evaluated in two open-label, single-arm ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Doxercalciferol Injection is a clear, colorless solution supplied in 2 mL amber glass vials as follows. Total Strength per Total Volume - Strength per mL - Vial Count per ...
-
17 PATIENT COUNSELING INFORMATIONHypercalcemia - Advise patients to contact a health care provider if they develop symptoms of elevated calcium (e.g. feeling tired, difficulty thinking clearly, loss of appetite, nausea ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELDoxercalciferol Injection 4 mcg/2 mL Single-Dose Vial - NDC 68083-300-01 - Container Label - Doxercalciferol Injection 4 mcg/2 mL Single-Dose Vial - NDC 68083-300-50 - Carton Label ...
-
INGREDIENTS AND APPEARANCEProduct Information