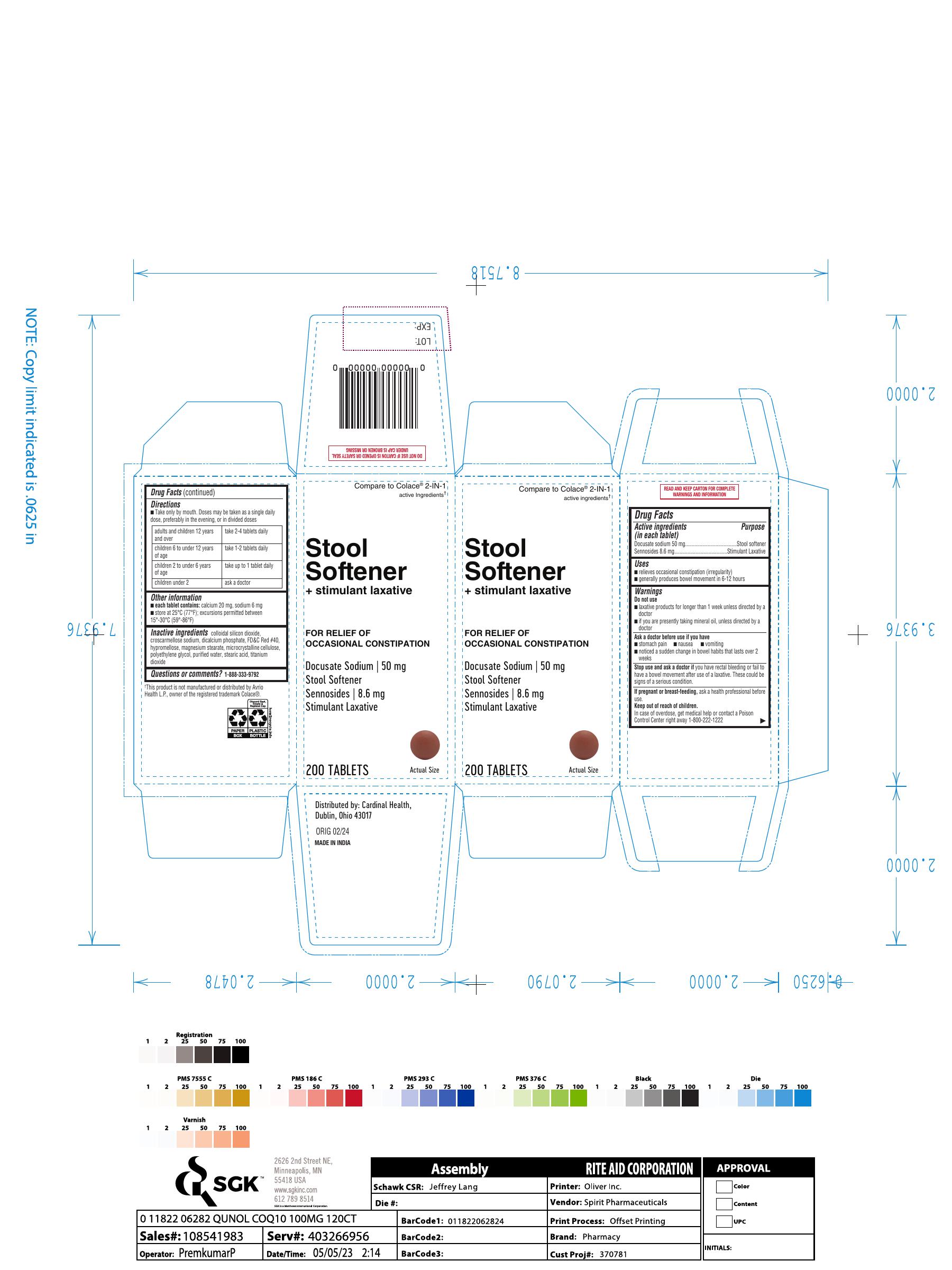
Label: STOOL SOFTENER PLUS STIMULANT LAXATIVE- docusate sodium,sennosides tablet
- NDC Code(s): 70000-0519-1
- Packager: Cardinal Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients (in each tablet)Docusate sodium 50 mg Sennosides 8.6 mg
- PurposeStool softener Stimulant Laxative
- Uses■ relieves occasional constipation (irregularity) ■ generally produces bowel movement in 6-12 hours
- Warnings Do not use ■ laxative products for longer than 1 week unless directed by a doctor ■ if you are presently taking mineral oil, unless directed by a doctor
- Ask a doctor before use if you have ■ stomach pain ■ nausea ■ vomiting ■ noticed a sudden change in bowel habits that lasts over 2 weeks
- Stop use and ask a doctorif you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
- If pregnant or breast-feeding,ask a health professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away 1-800-222-1222
- Directions ■ Take only by mouth. Doses may be taken as a single daily dose, preferably in the evening, or in divided doses adults and children 12 years and over take 2-4 tablets daily children 6 to under 12 years of age take 1-2 tablets daily children 2 to under 6 years of age take up to 1 tablet daily children under 2 ask a doctor
- Other information ■ each tablet contains:calcium 20 mg, sodium 6 mg ■ store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- Inactive ingredientscolloidal silicon dioxide, croscarmellose sodium, dicalcium phosphate, FD&C Red #40, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, purified water, stearic acid, titaniumdioxide
- Questions or comments?1-888-333-9792
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STOOL SOFTENER PLUS STIMULANT LAXATIVE
docusate sodium,sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0519 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FD&C RED NO. 40 (UNII: WZB9127XOA) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape ROUND Size 9mm Flavor Imprint Code S44 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0519-1 200 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 02/20/2024 Labeler - Cardinal Health (063997360)