Label: ULTRAMICROSIZE GRISEOFULVIN- griseofulvin tablet
- NDC Code(s): 62135-494-01, 62135-494-30, 62135-495-01, 62135-495-30
- Packager: Chartwell RX, LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONULTRAMICROSIZE GRISEOFULVIN TABLETS, USP - 125 MG; 250 MG - Rx only
-
DESCRIPTION
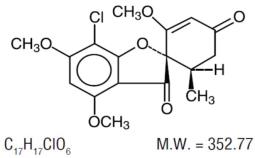
Ultramicrosize griseofulvin tablets, USP contain ultramicrosize crystals of griseofulvin, an antibiotic derived from a species of - Penicillium. Griseofulvin crystals are partly ...
-
CLINICAL PHARMACOLOGY
Microbiology - - Griseofulvin is fungistatic with - in vitro activity against various species of - Microsporum, Epidermophyton, and - Trichophyton. It ...
-
INDICATIONS AND USAGE
Ultramicrosize griseofulvin tablets are indicated for the treatment of the following ringworm infections; tinea corporis (ringworm of the body), tinea pedis (athlete’s foot), tinea cruris ...
-
CONTRAINDICATIONS
Two cases of conjoined twins have been reported since 1977 in patients taking griseofulvin during the first trimester of pregnancy. Griseofulvin should not be prescribed to pregnant patients. If ...
-
WARNINGS
Prophylactic Usage: Safety and efficacy of griseofulvin for prophylaxis of fungal infections have not been established.
-
Serious Skin Reactions
Severe skin reactions (e.g. Stevens-Johnson syndrome, toxic epidermal necrolysis) and erythema multiforme have been reported with griseofulvin use. These reactions may be serious and may result in ...
-
Hepatotoxicity
Elevations in AST, ALT, bilirubin, and jaundice have been reported with griseofulvin use. These reactions may be serious and may result in hospitalization or death. Patients should be monitored ...
-
PRECAUTIONS
Patients on prolonged therapy with any potent medication should be under close observation. Periodic monitoring of organ system function, including renal, hepatic, and hematopoietic, should be ...
-
ADVERSE REACTIONS
There have been post-marketing reports of severe skin and hepatic adverse events associated with griseofulvin use (see - WARNINGS section). When adverse reactions occur, they ...
-
DOSAGE AND ADMINISTRATION
Accurate diagnosis of infecting organism is essential. Identification should be made either by direct microscopic examination of a mounting of infected tissue in a solution of potassium hydroxide ...
-
HOW SUPPLIED
Ultramicrosize Griseofulvin Tablets, USP 125 mg, are supplied as off white, round tablets debossed with ‘CE’ over ‘3’ on one side and a functional score on the other side. They are available as ...
-
STORAGE
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP. Manufactured by: Chartwell Pharmaceuticals, LLC ...
-
PRINCIPAL DISPLAY PANEL
NDC 62135-494-01 - Ultramicrosize - Griseofulvin - Tablets, USP - 125 mg - Rx Only - 100 Tablets - Chartwell Rx ...
-
PRINCIPAL DISPLAY PANEL
NDC 62135-494-30 - Ultramicrosize - Griseofulvin - Tablets, USP - 125 mg - Rx Only - 30 Tablets - Chartwell Rx ...
-
PRINCIPAL DISPLAY PANEL
NDC 62135-495-01 - Ultramicrosize - Griseofulvin - Tablets, USP - 250 mg - Rx Only - 100 Tablets - Chartwell Rx ...
-
PRINCIPAL DISPLAY PANEL
NDC 62135-495-30 - Ultramicrosize - Griseofulvin - Tablets, USP - 250 mg - Rx Only - 30 Tablets - Chartwell Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information