Label: IFOSFAMIDE injection, powder, for solution
- NDC Code(s): 10019-927-01, 10019-927-20, 10019-929-03, 10019-929-60
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Ifosfamide for Injection safely and effectively. See full prescribing information for Ifosfamide for Injection.
Ifosfamide for injection, intravenous use
Initial U.S. Approval: 1988WARNING: MYELOSUPPRESSION, ENCEPHALOPATHY, NEPHROTOXICITY and UROTOXICITY
See full prescribing information for complete boxed warning.
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Ifosfamide for Injection is an alkylating drug indicated for use in adults in combination with certain other approved antineoplastic agents for third-line chemotherapy of germ cell testicular cancer. (1)
DOSAGE AND ADMINISTRATION
- •
- Administer Ifosfamide for Injection with extensive hydration consisting of at least 2 liters of oral or intravenous fluid per day to reduce the incidence or severity of bladder toxicity. (2.1, 5.3)
- •
- Administer mesna with Ifosfamide for Injection to reduce the incidence or severity of hemorrhagic cystitis. (2.1, 5.3)
- •
- Administer Ifosfamide for Injection as a slow intravenous infusion (at least 30 minutes) at a dose of 1.2 grams per m2 per day for 5 consecutive days. Repeat every 3 weeks or after recovery from hematologic toxicity. (2.2)
- •
- Individualize the dose and dosing schedule of Ifosfamide for Injection based on patient risk factors and adverse reactions. (2.2)
- •
- See Full Prescribing Information for instructions on preparation and administration. (2.3)
DOSAGE FORMS AND STRENGTHS
- •
- For injection: Single dose vials: 1 gram, 3 grams (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Myelosuppression: Monitor blood counts prior to treatment, during treatment, and as clinically indicated. (5.1)
- •
- Encephalopathy: Monitor for signs and symptoms of CNS toxicity during and after Ifosfamide for Injection treatment. Dose interruption or permanent discontinuation may be required based on individual safety and tolerability. (5.2)
- •
- Nephrotoxicity and Urotoxicity: Monitor signs and symptoms. Monitor serum and urine chemistries. (2.1, 5.3)
- •
- Cardiotoxicity: Arrhythmias, other ECG changes, and cardiomyopathy can occur and result in death. Cardiotoxicity is dose dependent and the risk is increased in patients with preexisting cardiac, treatment with other cardiotoxic agents, radiation, and renal impairment. (5.4)
- •
- Pulmonary toxicity: Interstitial pneumonitis, pulmonary fibrosis, and pulmonary toxicity with fatal outcomes can occur. Monitor for signs and symptoms of pulmonary toxicity and treat as clinically indicated (5.5)
- •
- Secondary malignancies can occur. (5.6)
- •
- Veno-occlusive Liver Disease can occur. (5.7)
- •
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise of potential risk to a fetus and use of effective contraception. (5.8, 8.1, 8.3)
- •
- Infertility: Can impair male and female reproductive function. (5.9)
- •
- Anaphylactic/anaphylactoid reactions have been reported. (5.10)
ADVERSE REACTIONS
The most common (≥ 10%) adverse reactions were alopecia, nausea/vomiting, hematuria, leukopenia, anemia, CNS toxicity, infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare at phone: 1 866 888 2472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: MYELOSUPPRESSION, ENCEPHALOPATHY, NEPHROTOXICITY and UROTOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Encephalopathy
5.3 Nephrotoxicity and Urotoxicity
5.4 Cardiotoxicity
5.5 Pulmonary Toxicity
5.6 Secondary Malignancies
5.7 Veno-occlusive Liver Disease
5.8 Embryo-Fetal Toxicity
5.9 Infertility
5.10 Anaphylactic/Anaphylactoid Reactions and Cross-sensitivity
5.11 Impairment of Wound Healing
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Inducers of CYP3A4
7.2 Inhibitors of CYP3A4
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Patients with Renal Impairment
8.7 Use in Patients with Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: MYELOSUPPRESSION, ENCEPHALOPATHY, NEPHROTOXICITY and UROTOXICITY
- •
- Myelosuppression can be severe and lead to fatal infections. Monitor blood counts prior to and at intervals after each treatment cycle [see Warnings and Precautions (5.1)].
- •
- Encephalopathy can be severe and may result in death. Monitor for CNS toxicity and discontinue treatment for encephalopathy [see Warnings and Precautions (5.2)].
- •
- Nephrotoxicity can be severe and result in renal failure. Hemorrhagic cystitis can be severe and can be reduced by the prophylactic use of mesna [see Warnings and Precautions (5.3)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Administer Ifosfamide for Injection with extensive hydration consisting of at least 2 liters of oral or intravenous fluid per day to reduce the incidence or severity of bladder toxicity.
Administer Ifosfamide for Injection with mesna to reduce the incidence or severity of hemorrhagic cystitis [see Warnings and Precautions (5.3)].
2.2 Recommended Dosage
The recommended dosage of Ifosfamide for Injection is 1.2 grams per m2 per day administered as a slow intravenous infusion (lasting at least 30 minutes) for 5 consecutive days. Treatment is repeated every 3 weeks or after recovery from hematologic toxicity.
Individualize the dose and dosing schedule of Ifosfamide for Injection based on patient risk factors and adverse reactions.
2.3 Preparation and Administration
Ifosfamide for Injection is a hazardous drug. Follow applicable special handling and disposal procedures. 1
Skin reactions associated with accidental exposure to Ifosfamide for Injection may occur. To minimize the risk of dermal exposure, always wear impervious gloves when handling vials and solutions containing Ifosfamide for Injection. If Ifosfamide for Injection solution contacts the skin or mucosa, immediately wash the skin thoroughly with soap and water or rinse the mucosa with copious amounts of water.
Prepare Ifosfamide for Injection for intravenous use by adding Sterile Water for Injection, USP or Bacteriostatic Water for Injection, USP (benzyl alcohol or parabens preserved) to the vial and shaking to dissolve. Before administration, the substance must be completely dissolved. Use the quantity of diluents shown in Table 1 below to reconstitute the product:
Table 1: Ifosfamide for Injection Quantities for Dilution and Final Concentrations Dosage Strength
Quantity of Diluent
Final Concentration
1 gram
20 mL
50 mg per mL
3 grams
60 mL
50 mg per mL
Solutions of ifosfamide may be diluted further to achieve concentrations of 0.6 to 20 mg/mL in the following fluids:
- •
- 5% Dextrose Injection, USP
- •
- 0.9% Sodium Chloride Injection, USP
- •
- Lactated Ringer’s Injections, USP
- •
- Sterile Water for Injection, USP
Because essentially identical stability results were obtained for Sterile Water admixtures as for the other admixtures (5% Dextrose Injection, 0.9% Sodium Chloride Injection, and Lactated Ringer’s Injection), the use of large volume parenteral glass bottles, VIAFLEX bags or PAB bags that contain intermediate concentrations or mixtures of excipients (e.g., 2.5% Dextrose Injection, 0.45% Sodium Chloride Injection, or 5% Dextrose and 0.9% Sodium Chloride Injection) is also acceptable.
Refrigerate constituted or constituted and further diluted solutions of Ifosfamide for Injection and use within 24 hours. Benzyl-alcohol-containing solutions can reduce the stability of ifosfamide.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Ifosfamide for Injection can cause myelosuppression that results in severe or fatal infections including sepsis or septic shock. Ifosfamide‑induced myelosuppression includes leukopenia, neutropenia, thrombocytopenia (with bleeding events), and anemia. The nadir of the leukocyte count usually occurs during the second week after administration of Ifosfamide for Injection. The risk of myelosuppression is dose‑dependent and increased in patients with reduced renal function, bone marrow metastases, prior radiation, and concomitant or prior therapy with other cytotoxic agents.
Monitor complete blood counts, including leukocytes, neutrophils, platelets, and hemoglobin prior to each administration of Ifosfamide for Injection, at appropriate intervals during treatment, and as clinically indicated.
Myelosuppression may require dosage delays. Avoid administration of Ifosfamide for Injection to patients with a WBC count below 2000/µL, a platelet count below 50,000/µL, or when signs or symptoms of active infection or severe immunosuppression are present.
5.2 Encephalopathy
Ifosfamide for Injection can cause encephalopathy which may be fatal. Signs and symptoms may include confusion, somnolence, coma, hallucination, blurred vision, psychotic behavior, extrapyramidal symptoms, urinary incontinence, and seizures.
Risk factors include high ifosfamide dosage, hypoalbuminemia, impaired renal function, poor performance status, bulky abdominal-pelvic disease, nephrotoxic treatments including cisplatin, CNS active drugs, or alcohol use.
Signs and symptoms may occur or recur within hours to days after administration of Ifosfamide for Injection. Continue supportive care until complete resolution of CNS signs and symptoms.
Monitor for signs and symptoms of encephalopathy during and after Ifosfamide for Injection treatment. Dose interruption or permanent discontinuation may be required based on individual safety and tolerability. Consider methylene blue for treatment of encephalopathy.
5.3 Nephrotoxicity and Urotoxicity
Ifosfamide for Injection can cause severe or fatal nephrotoxicity and urotoxicity including glomerular or tubular dysfunction, tubular necrosis, renal parenchymal necrosis, acute renal failure, chronic renal failure, and hemorrhagic cystitis (requiring blood transfusion).
Tubular damage may occur up to years after cessation of Ifosfamide for Injection treatment. The risk of nephrotoxicity is increased in patients with renal impairment or reduced nephron reserve. Hemorrhagic cystitis is dose‑dependent and the risk is increased with past or concomitant radiation of the bladder or busulfan treatment.
Evaluate glomerular and tubular kidney function before treatment with Ifosfamide for Injection, during Ifosfamide for Injection treatment, and as clinically indicated. Monitor serum and urine chemistries (including phosphorus and potassium) and urinary sediment for the presence of erythrocytes or other signs of nephrotoxicity. Signs and symptoms may include a decrease in glomerular filtration rate, increased serum creatinine, proteinuria, enzymuria, cylindruria, aminoaciduria, phosphaturia, glycosuria, osteomalacia, tubular acidosis, Fanconi syndrome, and syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Before starting treatment, exclude or correct any urinary tract obstructions [see Contraindications (4)]. During or immediately after administration, provide oral or intravenous fluid to force dieresis to reduce the risk of urinary tract toxicity. Administer mesna with Ifosfamide for Injection to reduce the incidence and severity of urotoxicity [see Dosage and Administration (2.1)].
Obtain a urinalysis prior to each dose of Ifosfamide for Injection. Avoid administration of Ifosfamide for Injection in patients with active urinary tract infections. If microscopic hematuria (greater than 10 RBCs per high power field) is present, then withhold administration of Ifosfamide for Injection until complete resolution. Further administration of Ifosfamide for Injection should be given with vigorous oral or parenteral hydration. Dosage interruption or permanent discontinuation may be required based on individual safety and tolerability.
5.4 Cardiotoxicity
Ifosfamide for Injection can cause severe or fatal cardiotoxicity including any of the following:
- •
- Supraventricular or ventricular arrhythmias, including atrial/supraventricular tachycardia, atrial fibrillation, pulseless ventricular tachycardia
- •
- Decreased QRS voltage and ST‑segment or T‑wave changes
- •
- Toxic cardiomyopathy leading to heart failure with congestion and hypotension
- •
- Pericardial effusion, fibrinous pericarditis, and epicardial fibrosis
Cardiotoxic effects are dose‑dependent and the risk is increased in patients with cardiac disease, prior or concomitant treatment with other cardiotoxic agents, radiation of the cardiac region, and renal impairment.
5.5 Pulmonary Toxicity
Ifosfamide for Injection can cause severe or fatal pulmonary toxicities including interstitial pneumonitis, pulmonary fibrosis, and respiratory failure. Monitor for signs and symptoms of pulmonary toxicity and treat as clinically indicated.
5.6 Secondary Malignancies
The incidence of secondary malignancies is increased in patients treated with Ifosfamide for Injection-containing regimens. Cases of myelodysplastic syndrome, acute leukemias, lymphomas, thyroid cancers, and sarcomas have occurred and may develop several years after chemotherapy has been discontinued.
5.7 Veno-occlusive Liver Disease
Veno-occlusive liver disease has been reported with chemotherapy that included ifosfamide.
5.8 Embryo-Fetal Toxicity
Based on mechanism of action and human and animal data, Ifosfamide for Injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1), Clinical Pharmacology (12.1) and Nonclinical Toxicology (13.1)]. Fetal growth retardation and neonatal anemia have been reported following exposure to ifosfamide‑containing chemotherapy regimens during pregnancy. Ifosfamide is genotoxic and mutagenic in male and female germ cells. Embryotoxic and teratogenic effects have been observed in mice, rats and rabbits at doses 0.05 to 0.075 times the human dose.
Advise pregnant women and females of reproductive potential of the potential risk to the fetus [see Use in Specific Populations (8.1)]. Verify the pregnancy status of females of reproductive potential prior to initiation of Ifosfamide for Injection. Advise females of reproductive potential to use effective contraception during treatment with Ifosfamide for Injection and for up to 12 months after completion of therapy.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with Ifosfamide for Injection and for 6 months after completion of therapy [see Use in Specific Populations (8.1, 8.3)].
5.9 Infertility
Male and female reproductive function and fertility may be impaired in patients treated with Ifosfamide for Injection. Ifosfamide interferes with oogenesis and spermatogenesis. Amenorrhea, azoospermia, and sterility in both sexes have been reported. Development of sterility appears to depend on the dose of ifosfamide, duration of therapy, and state of gonadal function at the time of treatment. Sterility may be irreversible in some patients. Advise patients on the potential risks for infertility [see Use in Specific Populations (8.3 and 8.4)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted from widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The adverse reactions in Table 2 below are based on 30 publications describing clinical experience with fractionated administration of ifosfamide as a single agent with a total dose of 4 to 12 g/m2 per course.
Table 2: Adverse Reactions in Patients Treated with Single Agent Ifosfamide for Injection - *
- Includes dysuria and pollakiuria
- †
- Includes acute renal failure, irreversible renal failure (fatal outcomes) serum creatinine increased, BUN increased, creatinine clearance decreased, metabolic acidosis, anuria, oliguria, glycosuria, hyponatremia, uremia, creatinine clearance increased
- ‡
- Includes acute tubular necrosis, renal parenchymal damage, enzymuria, cylindruria, proteinuria
- §
- Includes neutropenia, granulocytopenia, lymphopenia, and pancytopenia
- ¶
- Includes anemia and decrease in hemoglobin/hematocrit
- #
- Includes severe or fatal bleeding
- Þ
- Includes coma and death
- ß
- Includes abnormal behavior, affect lability aggression, agitation, anxiety, aphasia, asthenia, ataxia, cerebellar syndrome, cerebral function deficiency, cognitive disorder, coma, confusional state, convulsions, cranial nerve dysfunction, depressed state of consciousness, depression, disorientation, dizziness, electroencephalogram abnormal, encephalopathy, flat affect, hallucinations, headache, ideation, lethargy, memory impairment, mood change, motor dysfunction, muscle spasms, myoclonus, progressive loss of brainstem reflexes, psychotic reaction, restlessness, somnolence, tremor, urinary incontinence
- à
- Includes phlebitis and irritation of the venous walls
- è
- Includes granulocytopenic fever
- ð
- Includes increased serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), alkaline phosphatase, gamma-glutamyltransferase (GGT) and lactate dehydrogenase (LDH)
- ø
- Includes severe or fatal congestive heart failure, tachycardia, pulmonary edema
- ý
- Includes shock and death
Adverse Reaction
Single Agent Ifosfamide for Injection
%
(number of patients)Skin and Subcutaneous Tissue Disorders
Alopecia
90%
(540/603)Dermatitis
0.08%
(1/1317)Papular rash
0.08%
(1/1317)Gastrointestinal Disorders
Nausea/Vomiting
47%
(443/964)Diarrhea
0.7%
(9/1317)Stomatitis
0.3%
(4/1317)Renal and Urinary Disorders
Hemorrhagic cystitis*
Hematuria
- - without mesna
44%
(282/640)- - with mesna
21%
(33/155)Macrohematuria
- - without mesna
11%
(66/594)- - with mesna
5%
(5/97)Renal dysfunction†
--
Renal structural damage‡
--
Blood and Lymphatic System Disorders
Leukopenia§ (any)
--
Leukopenia
<1 x 103/µL44%
(267/614)Anemia¶
38%
(202/533)Thrombocytopenia# (any)
--
Thrombocytopenia, 50 x 103/µL
4.8%
(35/729)Nervous System Disorders
15%
(154/1001)Peripheral neuropathy
0.4%
(5/1317)Infections and Infestations
Infection
10%
(112/1128)General Disorders and Administration Site Conditions
Phlebitisà
2.8%
(37/1317)Neutropenic feverè
1%
(13/1317)Fatigue
0.3%
(4/1317)Malaise
Unable to calculate
Hepatobiliary Disorders
Hepatotoxicityð
1.8%
(22/1190)Metabolism and Nutrition Disorders
Anorexia
1.1%
(15/1317)Cardiac Disorders
Cardiotoxicityø
0.5%
(7/1317)Vascular Disorders
Hypotensioný
0.3%
(4/1317)6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Ifosfamide for Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic Disorders: agranulocytosis, febrile bone marrow aplasia, bone marrow failure, disseminated intravascular coagulation, hemolytic anemia, hemolytic febrile uremic syndrome, methemoglobinemia, neonatal anemia
Cardiac Disorders: cardiac arrest*, cardiac failure*, arrhythmia*, cardiomyopathy*, cardiotoxicity, cardiac shock, ejection fraction decreased*, myocardial infarction*, myocarditis*, ventricular fibrillation*, ventricular tachycardia*, angina pectoris, atrial fibrillation, atrial flutter, bradycardia, bundle branch block left, bundle branch block right, congestive cardiomyopathy, electrocardiogram ST – segment abnormal, electrocardiogram QRD complex abnormal, electrocardiogram T-wave inversion, myocardial depression, myocardial hemorrhage, left ventricular failure, premature atrial contractions, palpitations pericardial effusion, pericarditis, supraventricular extrasystoles, ventricular extrasystoles
Congenital Disorders: fetal growth retardation
Ear Disorders: deafness, hypoacusis, tinnitus, vertigo
Endocrine Disorder: SIADH
Eye Disorders: conjunctivitis, eye irritation, vision blurred, visual impairment
Gastrointestinal Disorders: abdominal pain, cecitis, colitis, constipation, enterocolitis, ileus, gastrointestinal hemorrhage, mucosal ulceration, pancreatitis, salivary hypersecretion
General Disorders and Administrative Site Conditions: multi‑organ failure*, chest pain, chills, injection/infusion site reactions (including erythema, inflammation, pain, pruritus, swelling, tenderness), edema, general physical deterioration, mucosal inflammation, pain, pyrexia
Hepatobiliary Disorders: hepatic failure*, hepatitis fulminant*, cholestasis, cytolytic hepatitis, portal vein thrombosis, veno‑occlusive liver disease
Immune System Disorders: anaphylactic reaction, angioedema, hypersensitivity reaction, immunosuppression, urticaria
Infections:
The following manifestations have been associated with myelosuppression and immunosuppression caused by ifosfamide: increased risk for and severity of infections†, pneumonias†, sepsis and septic shock (including fatal outcomes), as well as reactivation of latent infections, including viral hepatitis†, Pneumocystis jiroveci†, herpes zoster, Strongyloides, progressive multifocal leukoencephalopathy†, and other viral and fungal infections.
† Severe immunosuppression has led to serious, sometimes fatal, infections
Metabolic and Nutrition Disorders: hypocalcemia, hypokalemia, hypophosphatemia, hyperglycemia, metabolic acidosis, polydipsia, tumor lysis syndrome
Musculoskeletal and Connective Tissue Disorders: arthralgia, growth retardation, myalgia, muscle twitching, osteomalacia, pain in extremity, rhabdomyolysis, rickets
Neoplasms: secondary malignancies*, acute lymphocytic leukemia, acute myeloid leukemia, acute promyelocytic leukemia, myelodysplastic syndrome, Non‑Hodgkin’s lymphoma, renal cell carcinoma, sarcomas, thyroid cancer
Nervous System Disorders: seizure*, asterixis, dysarthria, dysesthesia, extrapyramidal disorder, fecal incontinence, gait disturbance hypothesia, leukoencephalopathy, movement disorder, neuralgia, paresthesia, polyneuropathy, reversible posterior leukoencephalopathy syndrome. Ifosfamide has been reintroduced after neurotoxicity. While some patients did not experience neurotoxicity, others had recurrent, including fatal, events.
Psychiatric Disorders: amnesia, bradyphrenia, catatonia, delirium, delusion, echolalia, logorrhea, mania mental status change, mutism, paranoia, panic attack, perseveration
Renal and Urinary Disorders: aminoaciduria, diabetes insipidus, enuresis, Fanconi syndrome, feeling of residual urine, nephrogenic phosphaturia, polyuria, tubulointerstitial nephritis
Reproductive System and Breast Disorders: amenorrhea, azoospermia, decreased blood estrogen, impairment of spermatogenesis, increased blood gonadotrophin, infertility, oligospermia, ovarian failure, ovulation disorder, premature menopause
Respiratory, Thoracic, and Mediastinal Disorders: acute respiratory distress syndrome*, pulmonary fibrosis*, pneumonitis*/interstitial lung disease*, pulmonary edema*, pulmonary hypertension*, respiratory failure*, alveolitis allergic, bronchospasm, cough, dyspnea, hypoxia, pleural effusion
Skin and Subcutaneous Disorders: erythema, facial swelling, hyperhidrosis, macular rash, nail disorder, palmar‑plantar erythrodysesthesia syndrome, petechiae, pruritus, radiation recall dermatitis, rash, skin hyperpigmentation, skin necrosis, Stevens‑Johnson syndrome, toxic epidermal necrolysis
Vascular Disorders: capillary leak syndrome, deep vein thrombosis, flushing, hypertension, pulmonary embolism, vasculitis
* Includes fatal outcomes
-
7 DRUG INTERACTIONS
7.1 Inducers of CYP3A4
CYP3A4 inducers may increase the metabolism of ifosfamide to its active alkylating metabolites. CYP3A4 inducers may increase the formation of the neurotoxic/nephrotoxic ifosfamide metabolite, chloroacetaldehyde. Closely monitor patients taking ifosfamide with CYP3A4 inducers for toxicities and consider dose adjustment.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on mechanism of action [see Clinical Pharmacology 12.1)], and human and animal data (see Data), Ifosfamide for Injection can cause fetal harm when administered to a pregnant woman. Fetal growth retardation and neonatal anemia have been reported following exposure to ifosfamide‑containing chemotherapy regimens during pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Animal studies indicate that ifosfamide is capable of causing gene mutations and chromosomal damage in vivo. In pregnant mice, resorptions increased and anomalies were present at day 19 after a 30 mg/m2 dose of ifosfamide was administered on day 11 of gestation. Embryo-lethal effects were observed in rats following the administration of 54 mg/m2 doses of ifosfamide from the 6th through the 15th day of gestation and embryotoxic effects were apparent after dams received 18 mg/m2 doses over the same dosing period. Ifosfamide is embryotoxic to rabbits receiving 88 mg/m2/day doses from the 6th through the 18th day after mating. The number of anomalies was also significantly increased over the control group.
8.2 Lactation
Risk Summary
Ifosfamide is excreted in breast milk. Because of the potential for serious adverse events, and the tumorigenicity shown for ifosfamide in animal studies, advise women not to breastfeed during treatment with Ifosfamide for Injection and for one week after the last dose.
8.3 Females and Males of Reproductive Potential
Ifosfamide for Injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating Ifosfamide for Injection [see Use in Specific Populations (8.1)].
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with Ifosfamide for Injection and for 12 months after the last dose.
Males
Advise males with female sexual partners of reproductive potential to use effective contraception during treatment with Ifosfamide for Injection and for 6 months after the last dose [see Nonclinical Toxicology (13.1)].
Infertility
Females
Amenorrhea has been reported in patients treated with ifosfamide. The risk of permanent chemotherapy induced amenorrhea increases with age.
Males
Men treated with ifosfamide may develop oligospermia or azoospermia. Azoospermia may be reversible in some patients, though the reversibility may not occur for several years after cessation of therapy. Sexual function and libido are generally unimpaired in these patients. Some degree of testicular atrophy may occur. Patients treated with ifosfamide have subsequently fathered children.
Females and Males
Based on animal studies, Ifosfamide for Injection may impair fertility in females and males of reproductive potential. The reversibility of these effects is unknown [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
Renal rickets and growth retardation in pediatric patients treated with ifosfamide have been reported.
Ifosfamide for Injection may cause temporary or permanent infertility in prepubertal girls or in females of child-bearing potential and may have an increased risk of developing premature menopause.
Ifosfamide for Injection may cause abnormal secondary sexual characteristic development in prepubertal males. Sterility, azoospermia, and testicular atrophy have been reported in male patients. Azoospermia may be reversible in some patients, but may not occur for several years after cessation of Ifosfamide for Injection therapy.
8.5 Geriatric Use
Clinical studies of Ifosfamide for Injection did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects.
A study of patients 40 to 71 years of age indicated that elimination half‑life appears to increase with advancing age [see Pharmacokinetics (12.3)]. This apparent increase in half‑life appeared to be related to increases in volume of distribution of ifosfamide with age. No significant changes in total plasma clearance or renal or non‑renal clearance with age were reported.
Ifosfamide and its metabolites are known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, closely monitor for adverse reactions and monitor renal function as clinically indicated.
8.6 Use in Patients with Renal Impairment
Ifosfamide and its metabolites are known to be excreted by the kidneys and may accumulate in plasma with decreased renal function. Closely monitor patients with renal impairment for adverse reactions and consider dosage modifications. Ifosfamide and its metabolites are dialyzable.
-
10 OVERDOSAGE
No specific antidote for Ifosfamide for Injection is known.
Patients who receive an overdose should be closely monitored for the development of toxicities. Serious consequences of overdosage include manifestations of dose-dependent toxicities such as CNS toxicity, nephrotoxicity, myelosuppression, and mucositis [see Warnings and Precautions (5)].
Management of overdosage would include general supportive measures to sustain the patient through any period of toxicity that might occur, including appropriate state-of-the-art treatment for any concurrent infection, myelosuppression, or other toxicity. Ifosfamide as well as ifosfamide metabolites are dialyzable.
Cystitis prophylaxis with mesna may be helpful in preventing or limiting urotoxic effects with overdose.
-
11 DESCRIPTION
Ifosfamide for Injection (ifosfamide for injection, USP) single-dose vials for constitution and administration by intravenous infusion each contain 1 gram or 3 grams of sterile ifosfamide.
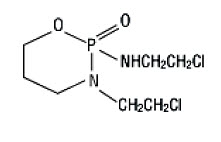
Ifosfamide is a alkylating drug chemically related to the nitrogen mustards and a synthetic analog of cyclophosphamide. Ifosfamide is 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide. The molecular formula is C7H15Cl2N2O2P and its molecular weight is 261.1. Ifosfamide is a white crystalline powder soluble in water. There are no excipients in the formulation. Each vial contains 1 gram or 3 grams of sterile ifosfamide alone.
Its structural formula is:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ifosfamide is a prodrug that requires metabolic activation by hepatic cytochrome P450 isoenzymes to exert its cytotoxic activity. Activation occurs by hydroxylation at the ring carbon atom forming the unstable intermediate 4‑hydroxyifosfamide and its ring-opened aldo tautomer, which decomposes to yield the cytotoxic and urotoxic compound acrolein and an alkylating isophosphoramide mustard.
The exact mechanism of action of ifosfamide has not been determined, but its cytotoxic action is primarily through DNA crosslinks caused by alkylation by the isophosphoramide mustard at guanine N-7 positions. The formation of inter- and intra-strand cross-links in the DNA results in cell death.
12.3 Pharmacokinetics
Ifosfamide exhibits dose-dependent pharmacokinetics in humans. At single doses of 3.8 to 5.0 g/m2, the plasma concentrations decay biphasically and the mean terminal elimination half-life is about 15 hours. At doses of 1.6 to 2.4 g/m2/day, the plasma decay is monoexponential and the terminal elimination half-life is about 7 hours.
Ifosfamide exhibits time-dependent pharmacokinetics in humans. Following intravenous administration of 1.5 g/m2 over 0.5 hour once daily for 5 days to 15 patients with neoplastic disease, a decrease in the median elimination half-life from 7.2 hour on Day 1 to 4.6 hours on Day 5 occurred with a concomitant increase in the median clearance from 66 mL/min on Day 1 to 115 mL/min on Day 5. There was no significant change in the volume of distribution on Day 5 compared with Day 1.
Distribution
Ifosfamide volume of distribution (Vd) approximates the total body water volume, suggesting that distribution takes place with minimal tissue binding. Following intravenous administration of 1.5 g/m2 over 0.5 hour once daily for 5 days to 15 patients with neoplastic disease, the median Vd of ifosfamide was 0.64 L/kg on Day 1 and 0.72 L/kg on Day 5. Ifosfamide shows little plasma protein binding. Ifosfamide and its active metabolites are extensively bound by red blood cells. Ifosfamide is not a substrate for P-glycoprotein.
Metabolism
Ifosfamide is extensively metabolized in humans through two metabolic pathways: ring oxidation (“activation”) to form the active metabolite, 4-hydroxy-ifosfamide and side-chain oxidation to form the inactive metabolites, 3-dechloro-ethylifosfamide or 2-dechloroethylifosfamide with liberation of the toxic metabolite, chloroacetaldehyde. Small quantities (nmol/mL) of ifosfamide mustard and 4‑hydroxyifosfamide are detectable in human plasma. Metabolism of ifosfamide is required for the generation of the biologically active species and while metabolism is extensive, it is also quite variable among patients.
Excretion
After administration of doses of 5 g/m2 of 14C-labeled ifosfamide, from 70% to 86% of the dosed radioactivity was recovered in urine as metabolites, with about 61% of the dose excreted as parent compound. At doses of 1.6 to 2.4 g/m2 only 12% to 18% of the dose was excreted in the urine as unchanged drug within 72 hours. Two different dechloroethylated derivatives of ifosfamide, 4-carboxyifosfamide, thiodiacetic acid and cysteine conjugates of chloroacetic acid have been identified as the major urinary metabolites of ifosfamide in humans and only small amounts of 4‑hydroxyifosfamide and acrolein are present.
Specific Populations
Pediatric Patients
Population PK analysis was performed on plasma data from 32 pediatric patients various malignant diseases aged between 1 and 18 years. Patients received a total of 45 courses of ifosfamide at doses of 1.2, 2.0 and 3.0 g/m2 given intravenously over 1 or 3 hours on 1, 2, or 3 days. The mean±standard error population estimates for the initial clearance and volume of distribution of ifosfamide were 2.4±0.33 L/h/m2 and 21±1.6 L/m2 with an interindividual variability of 43% and 32%, respectively.
Effect of Age
A study of 20 patients between 40 to 71 years of age receiving 1.5 g/m2 of ifosfamide daily for 3 or 5 days indicated that elimination half‑life appears to increase with age. The elimination half‑life increase appeared to be related to the increase in ifosfamide volume of distribution with age. No significant changes in total plasma clearance or renal clearance with age were reported.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ifosfamide has been shown to be carcinogenic in rats when administered by intraperitoneal injection at 6 mg/kg (37 mg/m2, or about 3% of the daily human dose on a mg/m2 basis) 3 times a week for 52 weeks. Female rats had a significantly higher incidence of uterine leiomyosarcomas and mammary fibroadenomas than vehicle controls.
The mutagenic potential of ifosfamide has been documented in bacterial systems in vitro and mammalian cells in vivo. In vivo, ifosfamide has induced mutagenic effects in mice and Drosophila melanogaster germ cells, and has induced a significant increase in dominant lethal mutations in male mice as well as recessive sex-linked lethal mutations in Drosophila.
Ifosfamide was administered to male and female beagle dogs at doses of 1.00 or 4.64 mg/kg/day (20 or 93 mg/m2) orally 6 days a week for 26 weeks. Male dogs at 4.64 mg/kg (about 7.7% of the daily clinical dose on a mg/m2 basis) had testicular atrophy with degeneration of the seminiferous tubular epithelium. In a second study, male and female rats were given 0, 25, 50, or 100 mg/kg (0, 150, 300, or 600 mg/m2) ifosfamide intraperitoneally once every 3 weeks for 6 months. Decreased spermatogenesis was observed in most male rats given 100 mg/kg (about half the daily clinical dose on a mg/m2 basis).
-
14 CLINICAL STUDIES
Patients with refractory testicular cancer (n=59) received a combination of ifosfamide, cisplatin, and either etoposide (VePesid) or vinblastine (VIP) as third-line therapy or later. The selection of etoposide or vinblastine (“V” in the VIP regimen) was guided by the therapeutic effect achieved with prior regimens. The contribution of ifosfamide to the VIP combination was determined in patients treated with cisplatin-etoposide prior to ifosfamide-cisplatin-etoposide or those who received cisplatin-vinblastine prior to ifosfamide-cisplatin-vinblastine.
A total of 59 patients received a third-line salvage regimen which consisted of ifosfamide 1.2 g/m2/day intravenously on days 1 to 5, cisplatin 20 mg/m2/day intravenously on days 1 to 5, and either etoposide 75 mg/m2/day intravenously on days 1 to 5 or vinblastine 0.22 mg/kg intravenously on day 1. Efficacy results with the VIP regimen were compared to data pooled from six single agent phase II trials conducted between August 1980 and October 1985 including a total of 90 patients of whom 65 were eligible as controls of this study. Twenty-three patients in the VIP regimen became free of disease with VIP alone or VIP plus surgery, whereas a single patient in the historical control group achieved complete response. The median survival time exceeded two years in the VIP group versus less than one year in the control group. Performance status ≥ 80, embryonal carcinoma and minimal disease were favorable prognostic factors for survival. In all prognostic categories, the difference between VIP and historical controls remained highly significant.
- *
- Gehan-Breslow and Mantel-Cox tests
Table 3: Efficacy Results
Number. (%) of Patients
VIP
Control
p-value
Total Patients
59 (100)
65 (100)
Disease-free
23 (39)
1 (2)
< 0.001
Chemotherapy alone
15 (25)
1 (2)
< 0.001
Chemotherapy plus surgery
8 (14)
0
Overall Response
32 (54)
2 (3)
< 0.001
Time to progression (weeks)
Median
19
4
< 0.001*
Range
1 – 205+
1 – 29
Disease-free interval (weeks)
Median
114
29
Range
13 – 205+
--
Survival (weeks)
Median
53
10
< 0.001*
Range
1 – 205+
1 – 123+
In a study, 50 fully evaluable patients with germ cell testicular cancer were treated with Ifosfamide for Injection in combination with cisplatin and either vinblastine or etoposide after failing (47 of 50 patients) at least two prior chemotherapy regimens consisting of cisplatin/vinblastine/bleomycin, (PVB), cisplatin/vinblastine/actinomycin D/bleomycin/cyclophosphamide, (VAB6), or the combination of cisplatin and etoposide. Patients were selected for remaining cisplatin sensitivity because they had previously responded to a cisplatin containing regimen and had not progressed while on the cisplatin containing regimen or within 3 weeks of stopping it. Patients served as their own control based on the premise that long term complete responses could not be achieved by retreatment with a regimen to which they had previously responded and subsequently relapsed.
Ten of 50 fully evaluable patients were still alive 2 to 5 years after treatment. Four of the 10 long term survivors were rendered free of cancer by surgical resection after treatment with the ifosfamide regimen; median survival for the entire group of 50 fully evaluable patients was 53 weeks.
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Myelosuppression
- •
- Advise patients that treatment with Ifosfamide for Injection may cause myelosuppression which can be severe and lead to infections and fatal outcomes.
- •
- Inform patients of the risks associated with the use of Ifosfamide for Injection and plan for regular blood monitoring during therapy [see Boxed Warning, Warnings, and Precautions (5.1)].
- •
- Inform patients to report fever or other symptoms of an infection [see Boxed Warning, Warnings and Precautions (5.1), Adverse Reactions (6.2)].
- •
- Advise patients on the risks of bleeding and anemia [see Warnings and Precautions (5.1, 5.8), Adverse Reactions (6.2)], Use in Specific Populations (8.1)].
Encephalopathy
- •
- Advise patients on the risk of encephalopathy and other neurotoxic effects with fatal outcome [see Boxed Warning, Warnings and Precautions (5.2)].
- •
- Inform patients that Ifosfamide for Injection may impair the ability to operate an automobile or other heavy machinery [see Boxed Warning, Warnings and Precautions (5.2)].
Nephrotoxicity and Urotoxicity
- •
- Advise patients on the risk of bladder and kidney toxicity.
- •
- Advise patients of the need to increase fluid intake and frequent voiding to prevent accumulation in the bladder [see Warnings and Precautions (5.3)].
Cardiotoxicity
- •
- Advise patients on the risk of cardiotoxicity and fatal outcome.
- •
- Advise patients to report preexisting cardiac disease and manifestations of cardiotoxicity [see Warnings and Precautions (5.4), Adverse Reactions (6.2)].
Pulmonary Toxicity
- •
- Advise patients on the risk of pulmonary toxicity leading to respiratory failure with fatal outcome.
- •
- Inform patients to report signs and symptoms of pulmonary toxicity [see Warnings and Precautions (5.5)].
Secondary Malignancies
- •
- Advise patients on the risk of secondary malignancies due to therapy [see Warnings and Precautions (5.6)].
Veno-occlusive Liver Disease
- •
- Advise patients on the risk of veno-occlusive liver disease [see Warnings and Precautions (5.7)].
Embryo-Fetal Toxicity
- •
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1)].
- •
- Advise females of reproductive potential to use effective contraception during treatment with Ifosfamide for Injection and for 12 months after the last dose [see Use in Specific Populations (8.3)].
- •
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment with Ifosfamide for Injection and for 6 months after the last dose [see Use in Specific Populations (8.3)].
Lactation
- •
- Advise women not to breastfeed during treatment with Ifosfamide for Injection and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Infertility
- •
- Advise females and males of reproductive potential that Ifosfamide for Injection may cause temporary or permanent infertility [see Use in Specific Populations (8.3)].
Skin and Subcutaneous Tissue Disorders
- •
- Advise patients on the risk of alopecia, wound healing, and other serious skin and subcutaneous tissue disorders [see Warnings and Precautions (5.11) Adverse Reactions (6.2)].
Gastrointestinal Disorders
- •
- Advise patients that the therapy may cause gastrointestinal disorders and alcohol may increase nausea and vomiting [see Adverse Reactions (6.2)].
- •
- Advise patients on the risk of stomatitis and the importance of proper oral hygiene [see Adverse Reactions (6.2)].
Eye Disorders
- •
- Advise patients on the risk of eye disorders such as visual impairment, blurred vision, and eye irritation [see Adverse Reactions (6.2)].
Ear and Labyrinth Disorders
- •
- Advise patients on the risk of ear and labyrinth disorders such as deafness, vertigo, and tinnitus [see Adverse Reactions (6.2)].
Manufactured by:
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
For Product Inquiry 1-800 ANA DRUG (1-800-262-3784)
Made in Germany
Novaplus Logo
NOVAPLUS is a registered trademark of Vizient, Inc.
Baxter and Viaflex are trademarks of Baxter International Inc.
PAB is a trademark of B Braun
HA-30-02-447
-
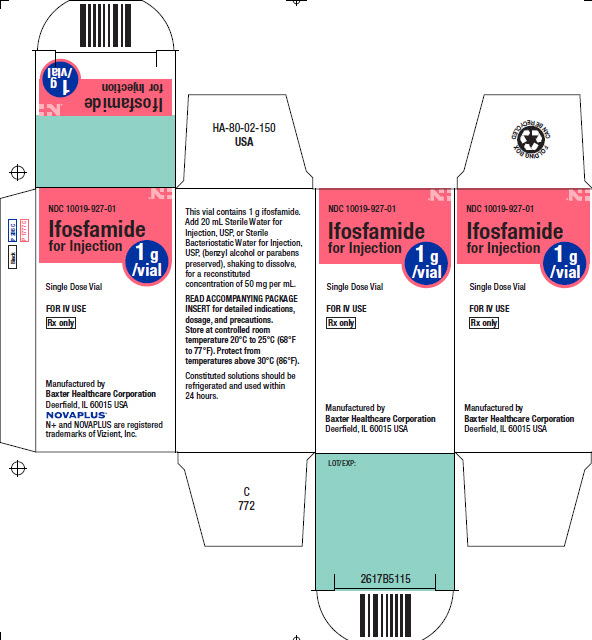
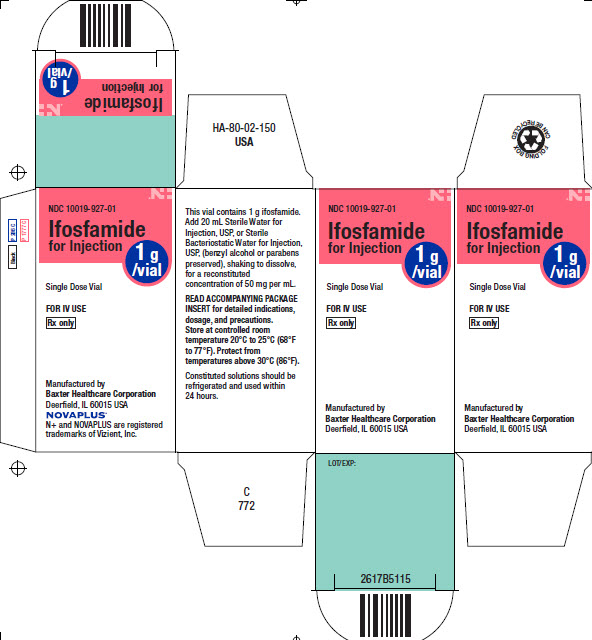
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Container Label
LOT/EXP:
Single Dose Vial
NDC 10019-927-20
Ifosfamide for
Injection1g
/vialRx only
FOR IV USE
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USANOVAPLUS Logo
N+ and NOVAPLUS are registered trademarks of Vizient, Inc.This vial contains 1 g ifosfamide.
Add 20 mL Sterile Water for Injection, USP, or
Sterile Bacteriostatic Water for Injection, USP,
(benzyl alcohol or parabens preserved), shaking
to dissolve, for a reconstituted concentration of
50 mg per mL.
Store at controlled room temperature 20°C to
25°C (68°F to 77°F). Protect from temperatures
above 30°C (86°F).
READ ACCOMPANYING PACKAGE INSERT for
detailed indications, dosage, and precautions.Bar code
(01)00310019927206
USA HA-65-01-560 C 780
Black
P 286 C
P 1777CIfosfamide
for Injection1g
/vialNDC 10019-927-01
Ifosfamide
for InjectionSingle Dose Vial
FOR IV USE
Rx onlyManufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USANOVAPLUS Logo
N+ and NOVAPLUS are registered
trademarks of Vizient, Inc.HA-80-02-150
USA
This vial contains 1 g ifosfamide.
Add 20 mL Sterile Water for
Injection, USP, or Sterile
Bacteriostatic Water for Injection,
USP, (benzyl alcohol or parabens
preserved), shaking to dissolve,
for a reconstituted
concentration of 50 mg per mL.READ ACCOMPANYING PACKAGE
INSERT for detailed indications,
dosage, and precautions.
Store at controlled room
temperature 20°C to 25°C (68°F
to 77°F). Protect from
temperatures above 30°C (86°F).
Constituted solutions should be
refrigerated and used within
24 hours.C
772NDC 10019-927-01
Ifosfamide
for Injection1g
/vialSingle Dose Vial
FOR IV USE
Rx onlyManufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USALOT/EXP:
2617B5115
Bar code
FOLDING BOX CAN BE RECYCLED Logo
NDC 10019-927-01
Ifosfamide
for Injection1g
/vialSingle Dose Vial
FOR IV USE
Rx onlyManufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USALOT/EXP:
Single Dose Vial
NDC 10019-929-60
Ifosfamide for
Injection3g
/vialRx only
FOR IV USE
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USANOVAPLUS Logo
N+ and NOVAPLUS are registered trademarks of Vizient, Inc.This vial contains 3 g ifosfamide.
Add 60 mL Sterile Water for Injection, USP, or
Sterile Bacteriostatic Water for Injection, USP,
(benzyl alcohol or parabens preserved), shaking
to dissolve, for a reconstituted concentration of
50 mg per mL.
Store at controlled room temperature 20°C to
25°C (68°F to 77°F). Protect from temperatures
above 30°C (86°F).
READ ACCOMPANYING PACKAGE INSERT for
detailed indications, dosage, and precautions.Bar code
(01)00310019929606
USA HA-65-01-563 C 781
Black
P 286 C
P 2705 CIfosfamide
for Injection3g
/vialNDC 10019-929-03
Ifosfamide
for Injection3g
/vialSingle Dose Vial
FOR IV USE
Rx onlyManufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USAHA-80-02-153
USA
This vial contains 3 g ifosfamide.
Add 60 mL Sterile Water for
Injection, USP, or Sterile
Bacteriostatic Water for Injection,
USP, (benzyl alcohol or parabens
preserved), shaking to dissolve,
for a reconstituted
concentration of 50 mg per mL.READ ACCOMPANYING PACKAGE
INSERT for detailed indications,
dosage, and precautions.Store at controlled room
temperature 20°C to 25°C (68°F
to 77°F). Protect from
temperatures above 30°C (86°F).Constituted solutions should be
refrigerated and used within
24 hours.C
775NDC 10019-929-03
Ifosfamide
for Injection3g
/vialSingle Dose Vial
FOR IV USE
Rx onlyManufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
NOVAPLUS Logo
N+ and NOVAPLUS are registered
trademarks of Vizient, Inc.LOT/EXP:
2617B5116
Bar code
FOLDING BOX CAN BE RECYCLED Logo
NDC 10019-929-03
Ifosfamide
for Injection3g
/vialSingle Dose Vial
FOR IV USE
Rx onlyManufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
NOVAPLUS Logo
N+ and NOVAPLUS are registered
trademarks of Vizient, Inc. -
INGREDIENTS AND APPEARANCE
IFOSFAMIDE
ifosfamide injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10019-927 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IFOSFAMIDE (UNII: UM20QQM95Y) (IFOSFAMIDE - UNII:UM20QQM95Y) IFOSFAMIDE 1 g in 20 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10019-927-01 1 in 1 CARTON 08/02/2018 1 NDC:10019-927-20 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019763 12/30/1988 IFOSFAMIDE
ifosfamide injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10019-929 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IFOSFAMIDE (UNII: UM20QQM95Y) (IFOSFAMIDE - UNII:UM20QQM95Y) IFOSFAMIDE 3 g in 60 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10019-929-03 1 in 1 CARTON 08/02/2018 1 NDC:10019-929-60 60 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019763 12/30/1988 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Deutschland GmbH 312520353 API MANUFACTURE(10019-927, 10019-929)