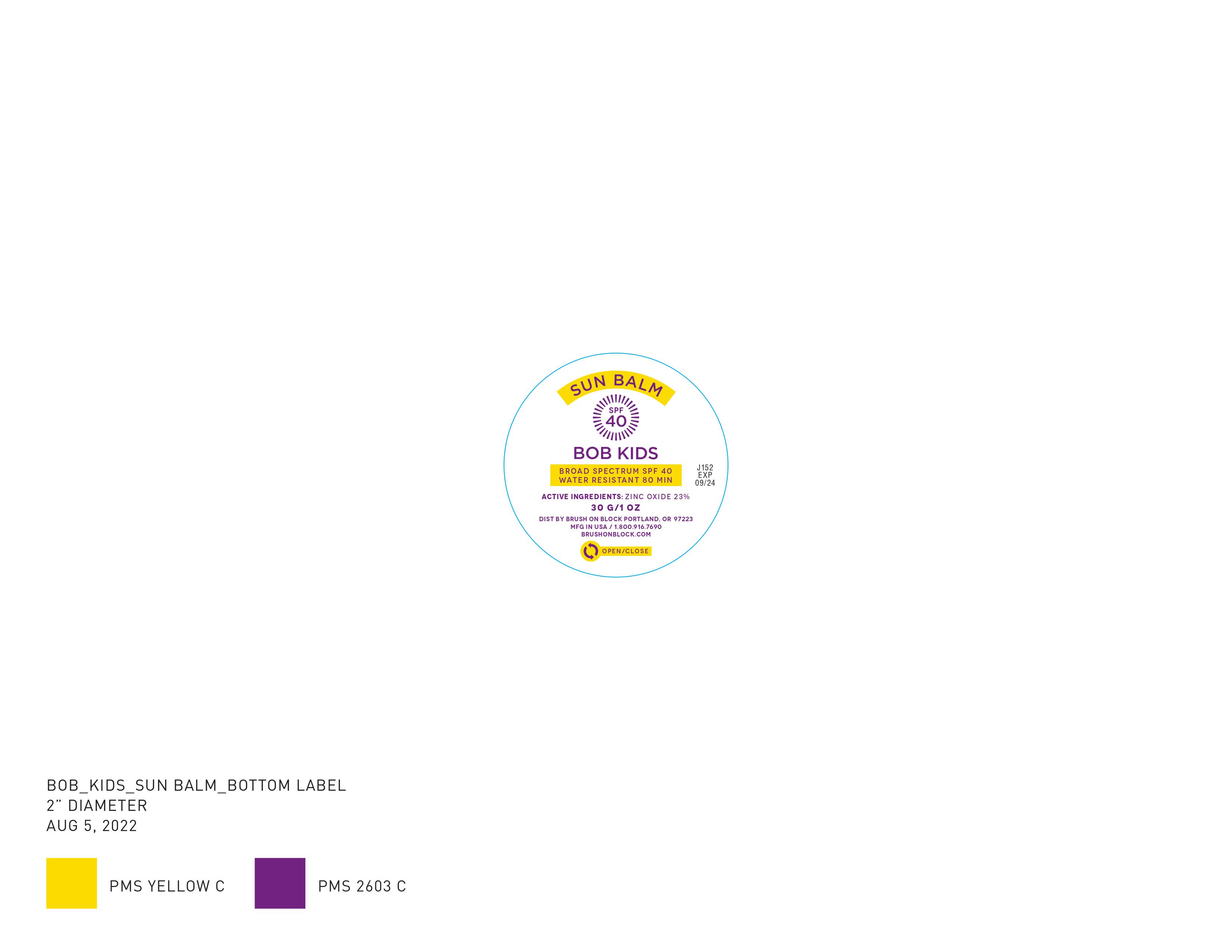
Label: BOB KIDS SUN BALM SPF 40- zinc oxide 23% cream
- NDC Code(s): 58274-009-01
- Packager: SPF Ventures, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply: after 80 minutes of swimming or sweating; immediately after towel drying; at least every 2 hours
- Children under 6 months: Ask a doctor.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m., wear long-sleeve shirts, pants, hats, and sunglasses.
-
Inactive Ingredients
Argan Oil, Behenyl Behenate, Bidens Pilosa Extract, Bisabolol, Butyloctyl Salicylate, C9-12 Alkane, Camellia Seed Oil, Candelilla Wax, Caprylic/Capric Triglyceride, Capryloyl Glycerin/Sebacic Acid Copolymer, Coco-Caprylate/Caprate, Cottonseed Oil, Flaxseed Oil, Frankincense Extract, Green Tea Leaf Extract, Hemp Seed Oil, Lauroyl Lysine, Meadowfoam Seed Oil, Mica, Murumuru Seed Butter, Polyhydroxystearic Acid, Polyurethane-100, Rice Bran Wax, Shea Butter, Silica, Sunflower Seed Oil, Triethoxycaprylylsilane, Triheptanoin, Tocopheryl Acetate.
- Other Information
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BOB KIDS SUN BALM SPF 40
zinc oxide 23% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58274-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 23 g in 100 g Inactive Ingredients Ingredient Name Strength ARGAN OIL (UNII: 4V59G5UW9X) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) LEVOMENOL (UNII: 24WE03BX2T) ASTROCARYUM MURUMURU SEED BUTTER (UNII: 12V64UPU6R) BEHENYL BEHENATE (UNII: K8NU647RJ0) BIDENS PILOSA LEAF (UNII: 457932TMZ8) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SHEA BUTTER (UNII: K49155WL9Y) CAMELLIA OLEIFERA SEED (UNII: 59ED29FM2J) CANDELILLA WAX (UNII: WL0328HX19) SUNFLOWER OIL (UNII: 3W1JG795YI) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) RICE BRAN (UNII: R60QEP13IC) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) TRIHEPTANOIN (UNII: 2P6O7CFW5K) LEVANT COTTONSEED OIL (UNII: N5CFT140R8) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) COCONUT ALKANES (UNII: 1E5KJY107T) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) LINSEED OIL (UNII: 84XB4DV00W) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58274-009-01 1 in 1 BOX 03/20/2023 1 3.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/20/2023 Labeler - SPF Ventures, LLC (055483891)