Label: ADYNOVATE (antihemophilic factor- recombinant pegylated kit
- NDC Code(s): 0944-4137-01, 0944-4138-01, 0944-4139-01, 0944-4140-01, view more
- Packager: Takeda Pharmaceuticals Amercia, Inc.
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADYNOVATE® safely and effectively. See full prescribing information for ADYNOVATE. ADYNOVATE, (Antihemophilic Factor, Recombinant ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEADYNOVATE®, Antihemophilic Factor (Recombinant), PEGylated, is a human antihemophilic factor indicated in children and adults with hemophilia A (congenital factor VIII deficiency) for: On-demand ...
-
2 DOSAGE AND ADMINISTRATIONFor intravenous use after reconstitution only. 2.1 Dose - Each vial label of ADYNOVATE states the actual factor VIII potency in international units. This may be more or less than the nominal ...
-
3 DOSAGE FORMS AND STRENGTHSADYNOVATE is a lyophilized powder in single-dose vials containing nominally (approximately) 250, 500, 750, 1000, 1500, 2000, and 3000 International Units (IU, units). The 250-1500 IU strengths ...
-
4 CONTRAINDICATIONSADYNOVATE is contraindicated in patients who have had prior anaphylactic reaction to ADYNOVATE, to the parent molecule (ADVATE®), mouse or hamster protein, or excipients of ADYNOVATE (e.g. Tris ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions including anaphylaxis, have been reported with ADYNOVATE. Hypersensitivity reactions that can progress to anaphylaxis may include ...
-
6 ADVERSE REACTIONSThe most common adverse reactions (≥1% of subjects) reported in the clinical studies were headache, diarrhea, rash, nausea, dizziness and urticaria. 6.1 Clinical Trials Experience - Because ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data with ADYNOVATE use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with ADYNOVATE. It ...
-
11 DESCRIPTIONADYNOVATE, Antihemophilic Factor (Recombinant), PEGylated, is formulated as a sterile, non-pyrogenic, white to off-white lyophilized powder for reconstitution for intravenous injection. The ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - ADYNOVATE, a PEGylated form of recombinant antihemophilic factor (ADVATE), [see Description (11)], temporarily replaces the missing coagulation factor VIII needed for ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate the carcinogenic potential of ADYNOVATE or studies to determine the effects of ADYNOVATE on ...
-
14 CLINICAL STUDIESOriginal Safety and Efficacy Clinical Trial - The safety, efficacy, and PK of ADYNOVATE were evaluated in a multicenter, open-label, prospective, non-randomized, two-arm clinical trial that ...
-
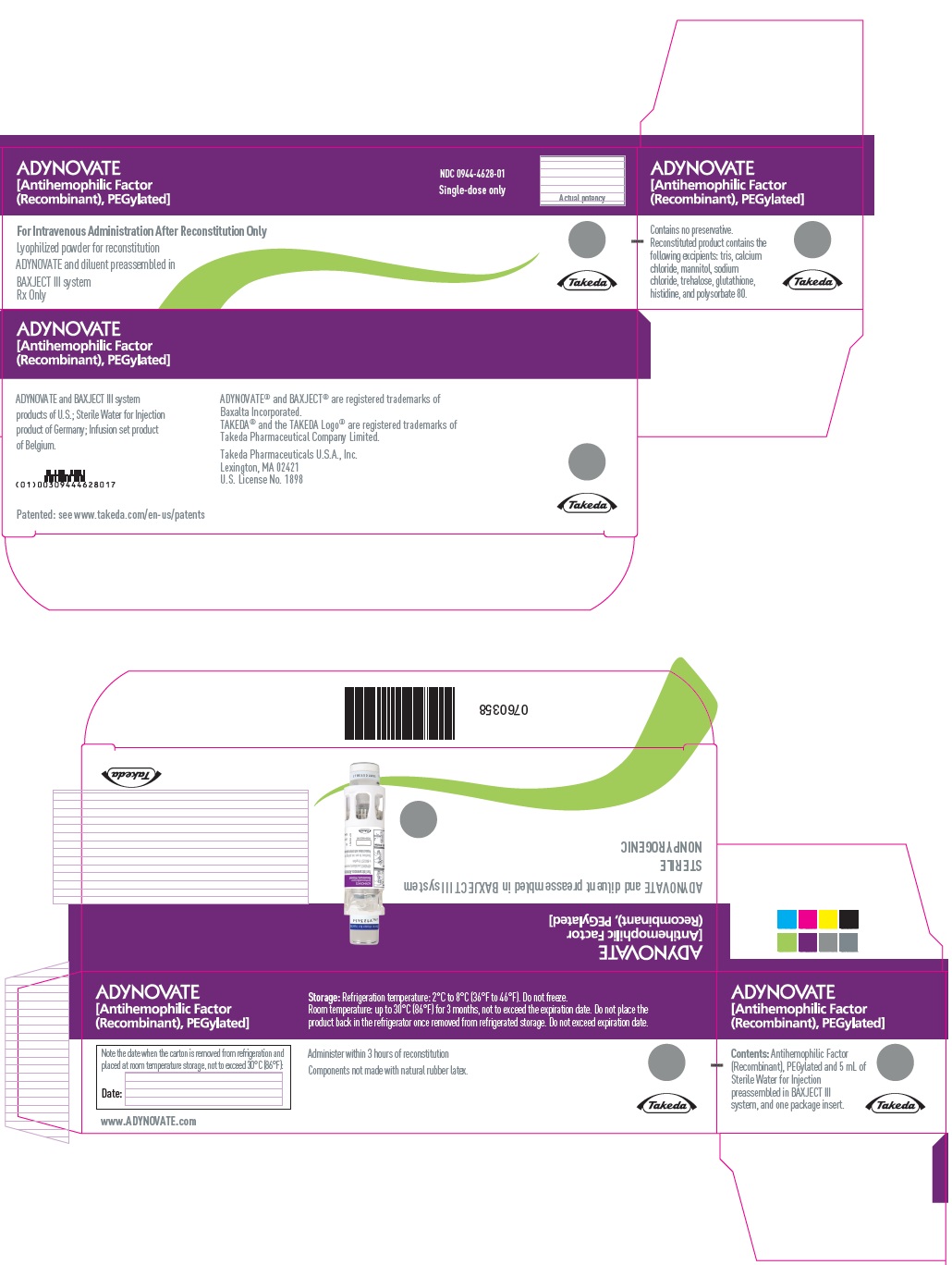
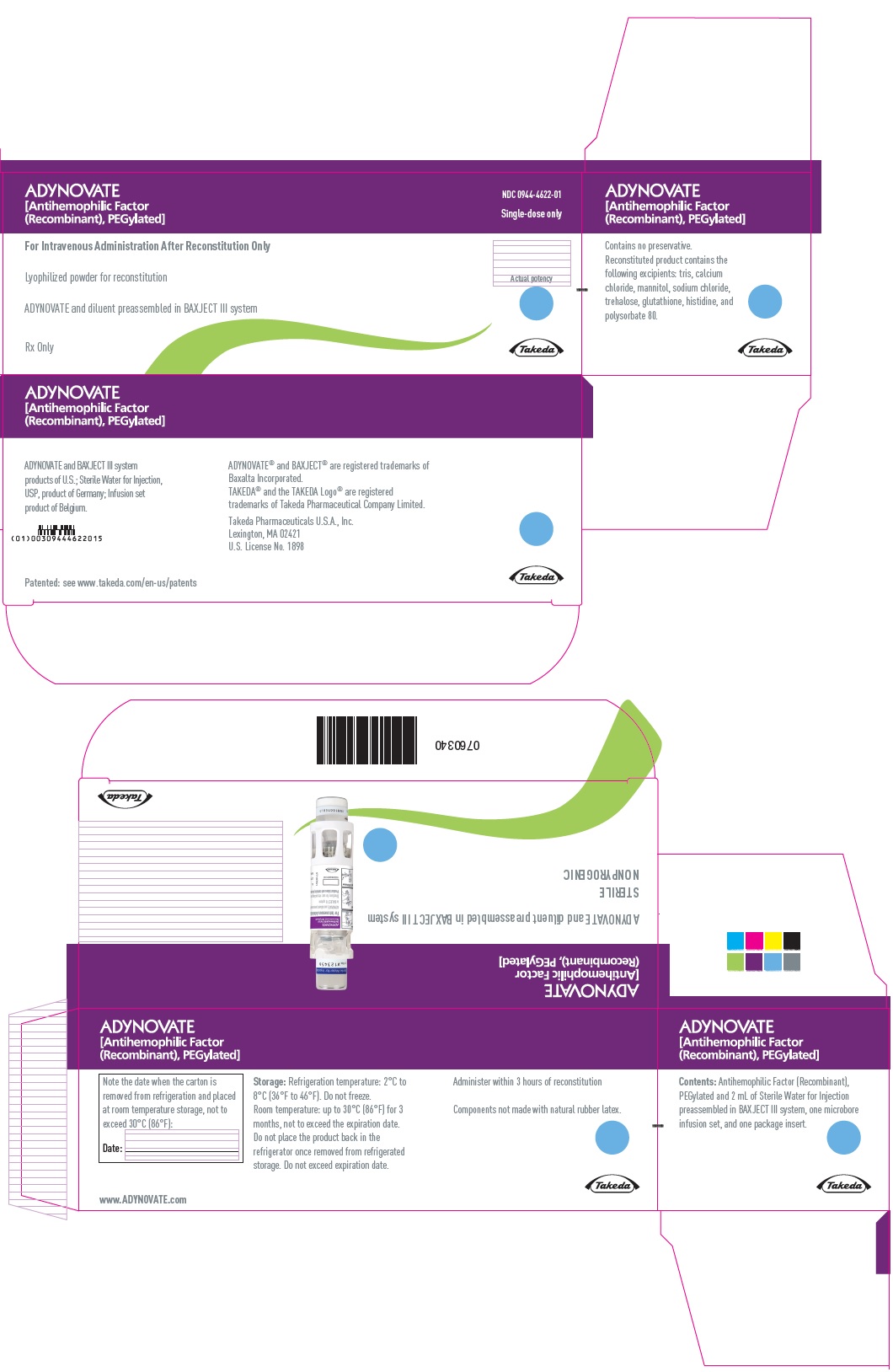
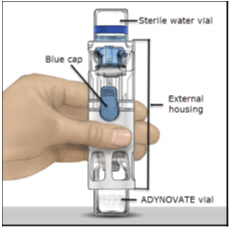
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - ADYNOVATE in a BAXJECT III system is packaged with 2 mL or 5 mL of Sterile Water for Injection, one Terumo Microbore Infusion set (2 mL only), one full prescribing physician ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patients to: Read the FDA-approved patient labeling (Patient Information and Instructions for Use). Call their healthcare provider or go to the emergency department right away if a ...
-
SPL UNCLASSIFIED SECTIONTakeda Pharmaceuticals U.S.A., Inc. Cambridge, MA 02142 - U.S. License No. 1898 - ADYNOVATE, ADVATE and BAXJECT are registered trademarks of Baxalta Incorporated. Takeda and are registered ...
-
FDA-Approved Patient LabelingPatient InformationADYNOVATE® [Antihemophilic Factor (Recombinant), PEGylated] This leaflet summarizes important information about ADYNOVATE. Please read it carefully before using this medicine. This information ...
-
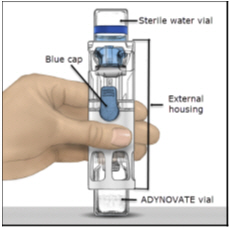
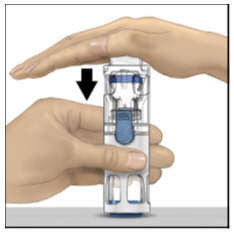
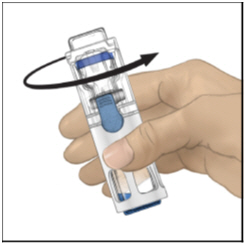
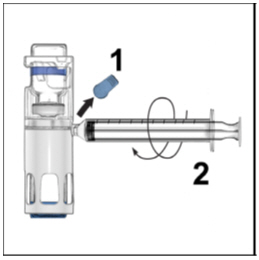
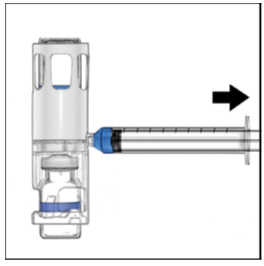
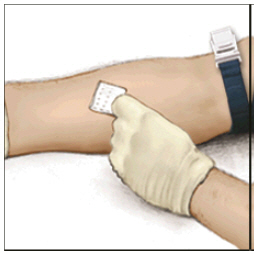
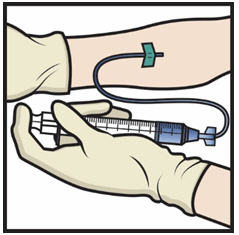
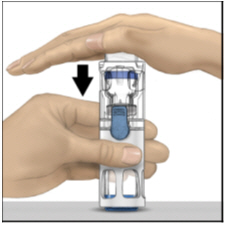
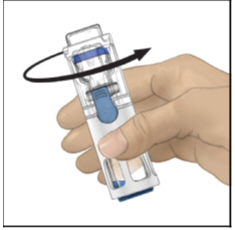
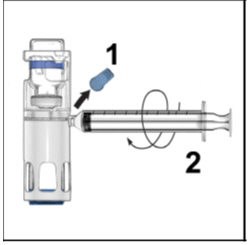
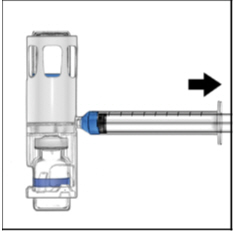
FDA-Approved Patient LabelingInstructions for UseADYNOVATE® [Antihemophilic Factor (Recombinant), PEGylated] (For intravenous use only) Do not attempt to do an infusion to yourself unless you have been taught how by your healthcare provider ...
-
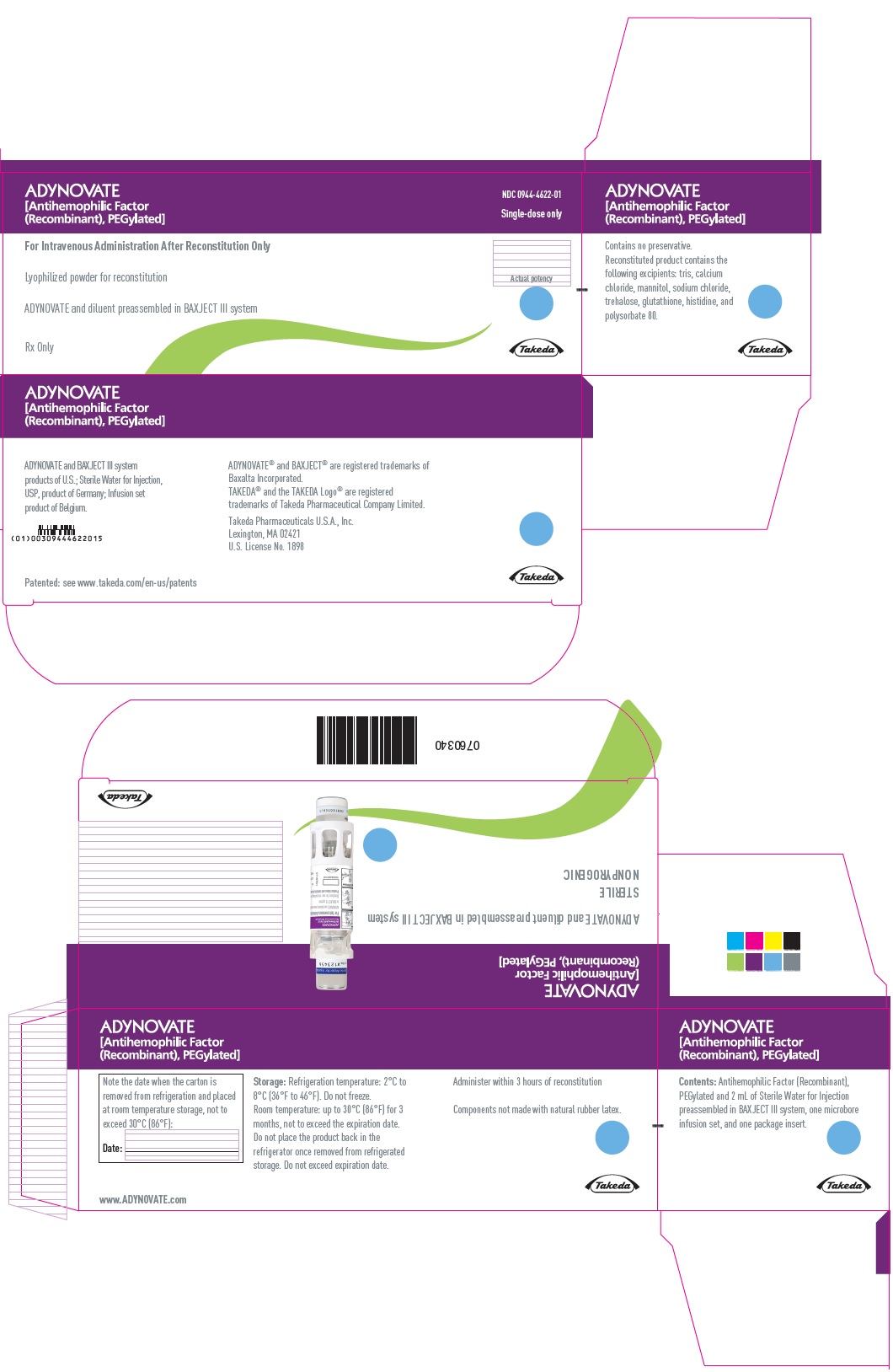
PRINCIPAL DISPLAY PANEL - Kit Carton - 250 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4622-01 - Single-dose only - For Intravenous Administration After Reconstitution Only - Lyophilized powder for ...
-
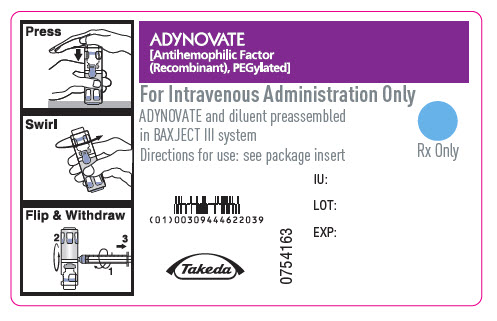
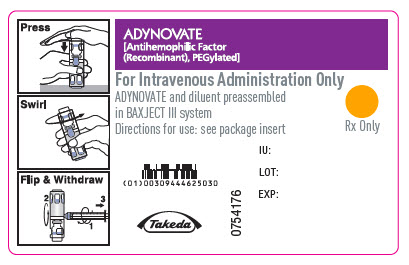
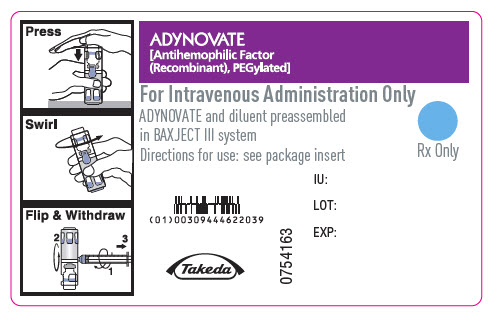
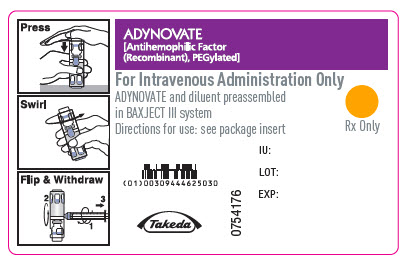
PRINCIPAL DISPLAY PANEL - Barrel Label - 250 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] For Intravenous Administration Only - ADYNOVATE and diluent preassembled - in BAXJECT® III system - Directions for use: see package ...
-
PRINCIPAL DISPLAY PANEL - Blister Label - 250 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4622-02 - Single-dose only - 6521479 - For Intravenous Administration Only - ADYNOVATE and diluent preassembled in BAXJECT ...
-
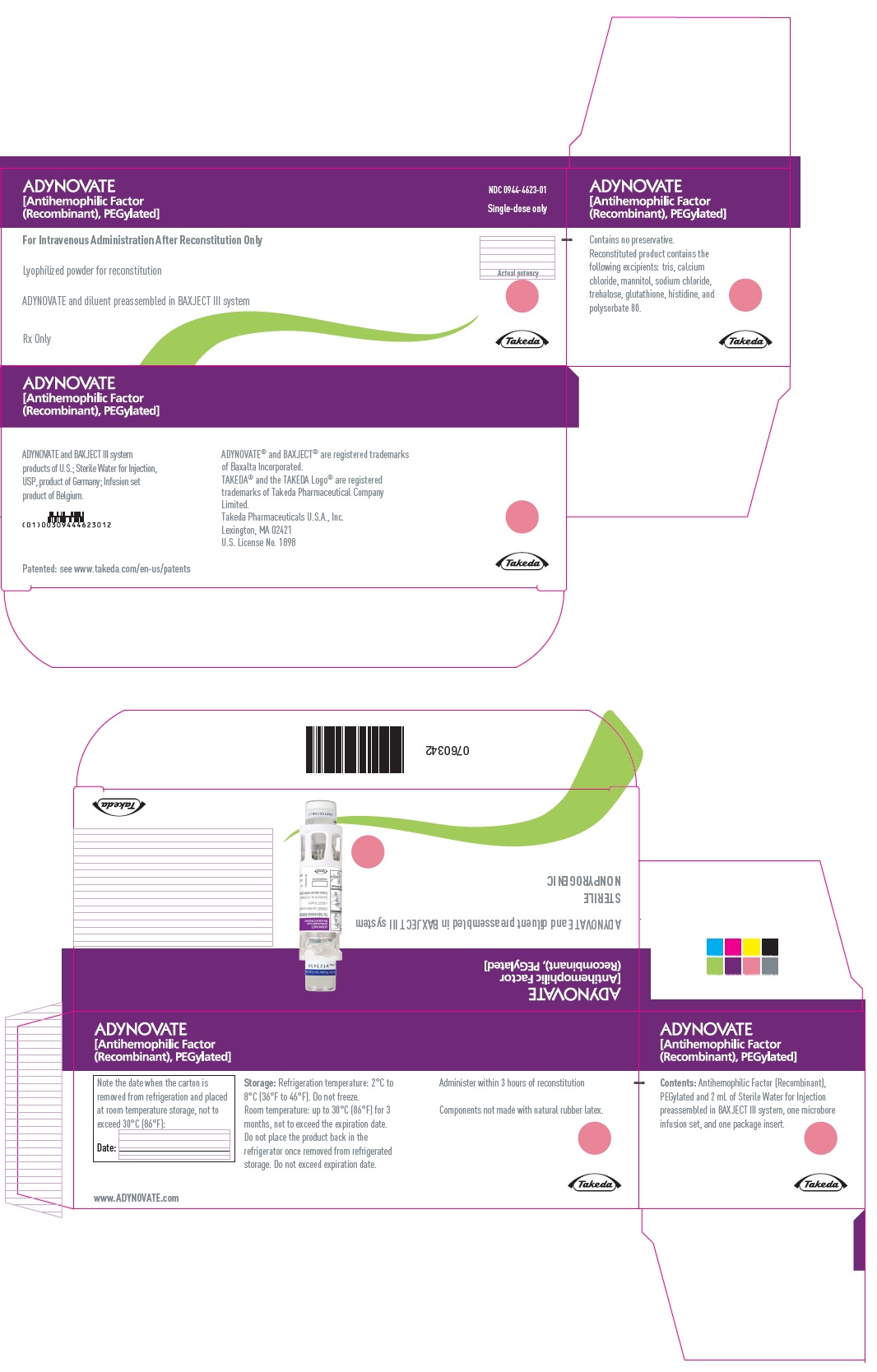
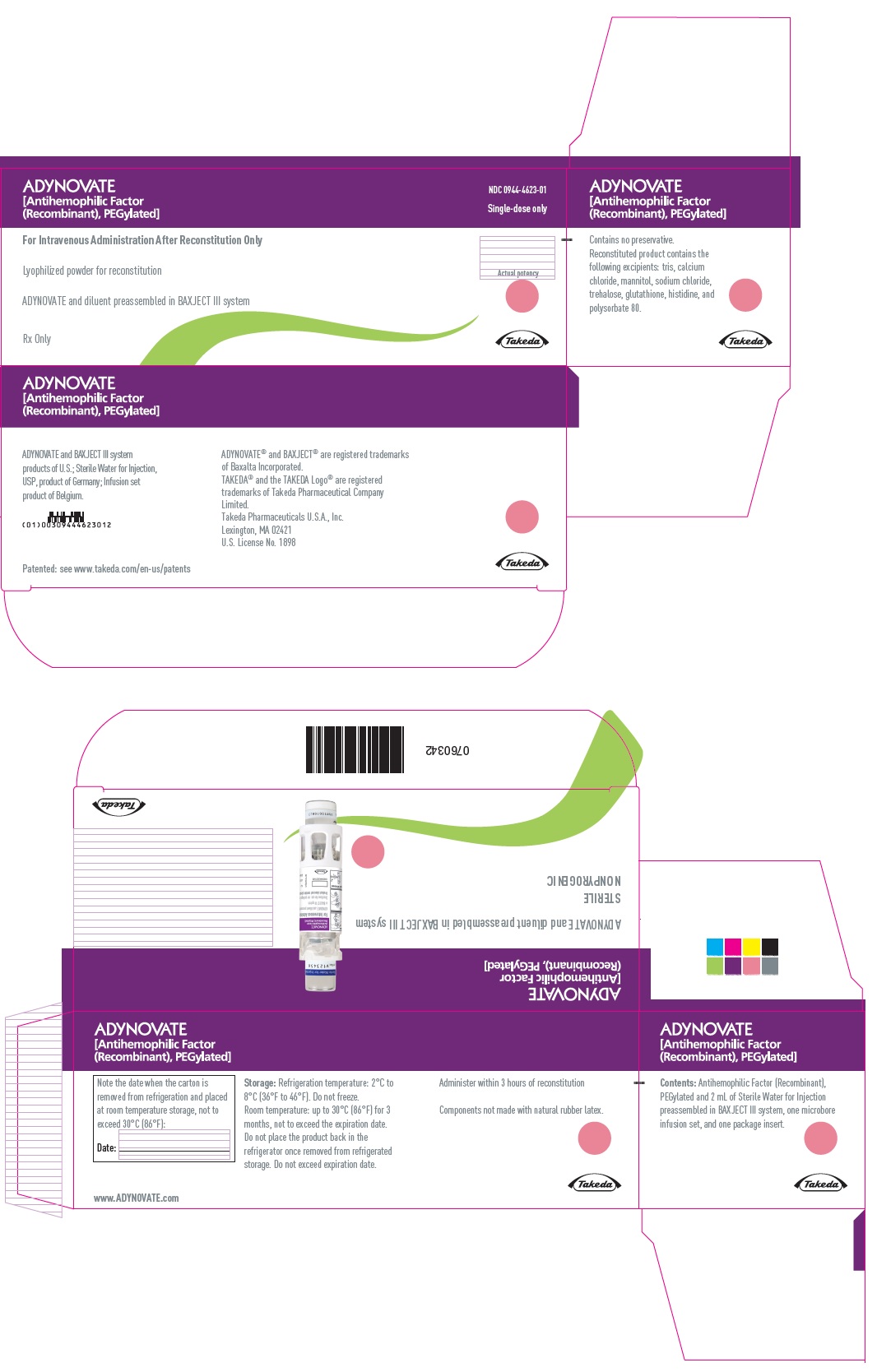
PRINCIPAL DISPLAY PANEL - Kit Carton - 500 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4623-01 - Single-dose only - For Intravenous Administration After Reconstitution Only - Lyophilized powder for ...
-
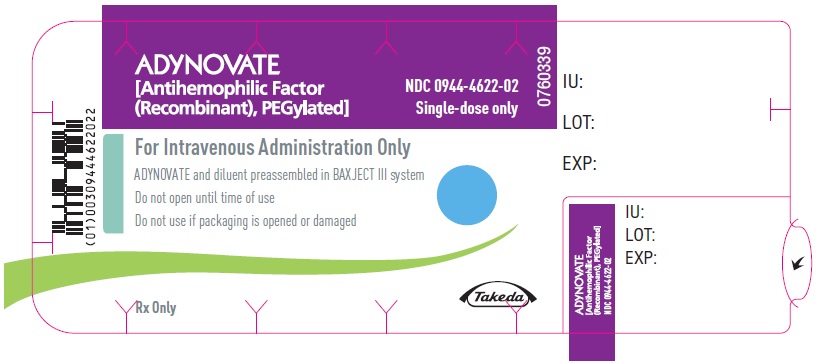
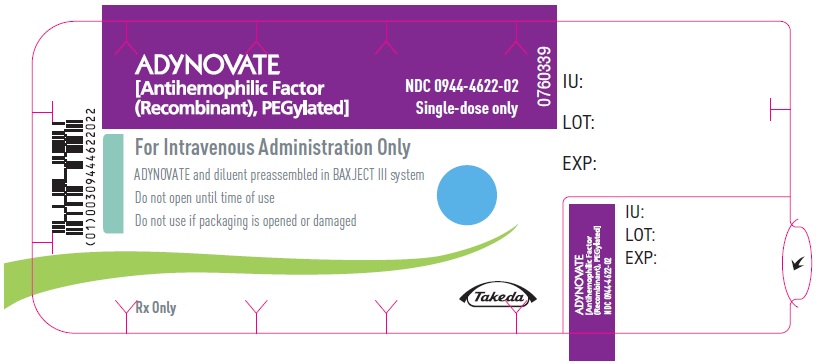
PRINCIPAL DISPLAY PANEL - Barrel Label - 500 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] For Intravenous Administration Only - ADYNOVATE and diluent preassembled - in BAXJECT® III system - Directions for use: see package ...
-
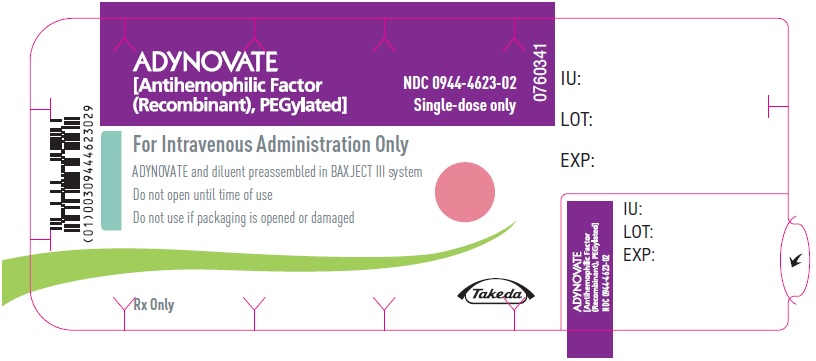
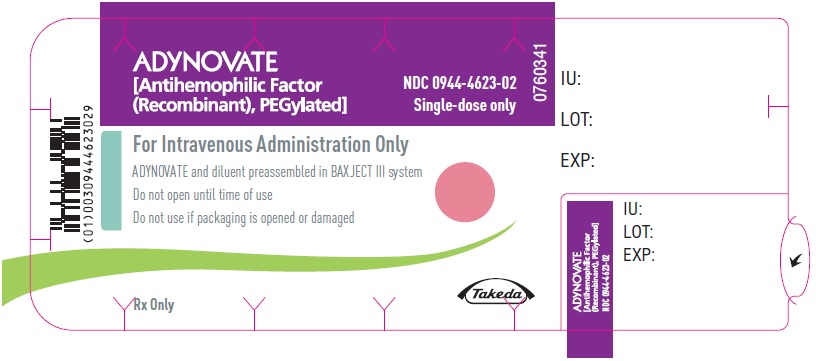
PRINCIPAL DISPLAY PANEL - Blister Label - 500 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4623-02 - Single-dose only - 6521484 - For Intravenous Administration Only - ADYNOVATE and diluent preassembled in BAXJECT ...
-
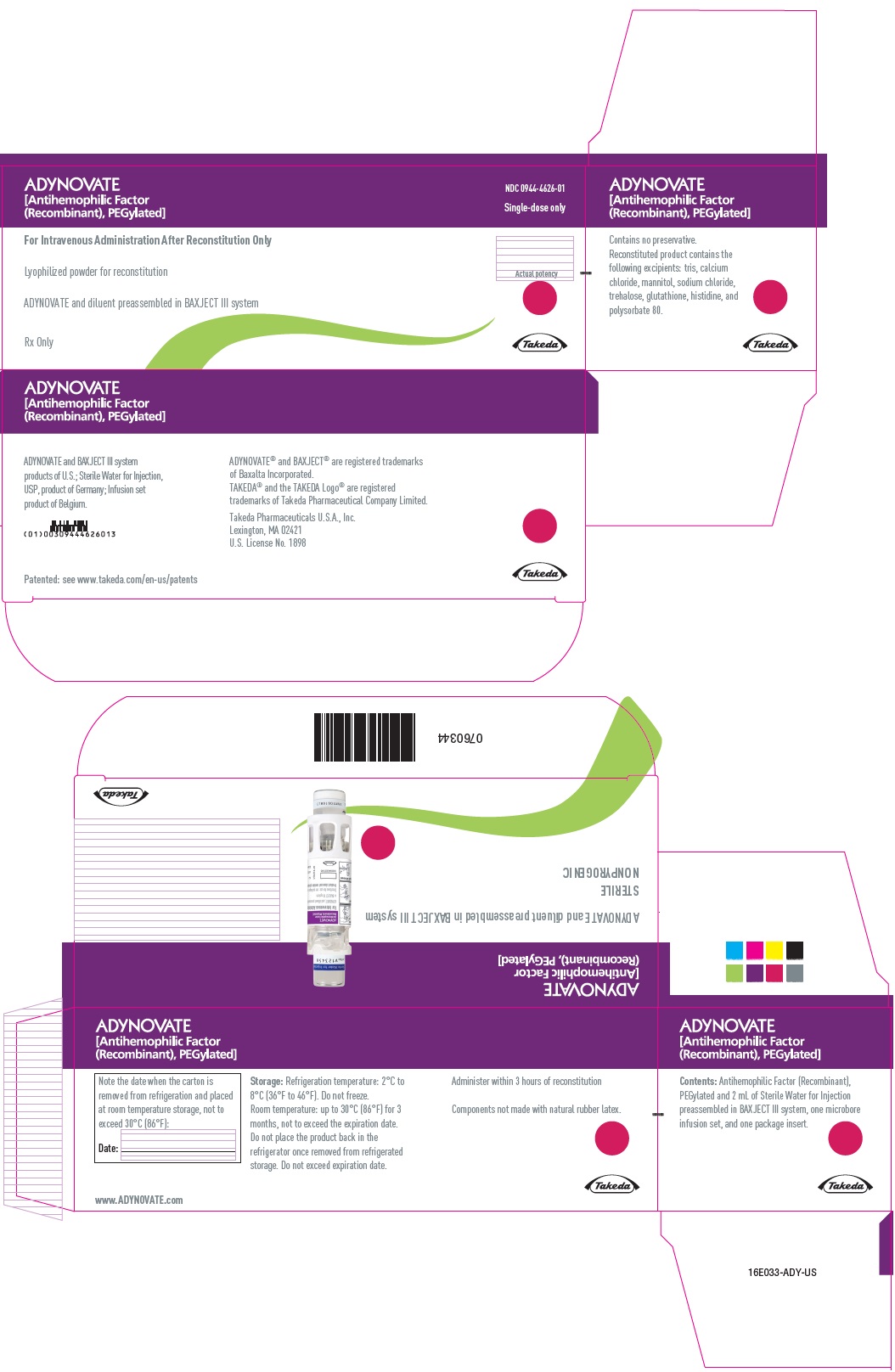
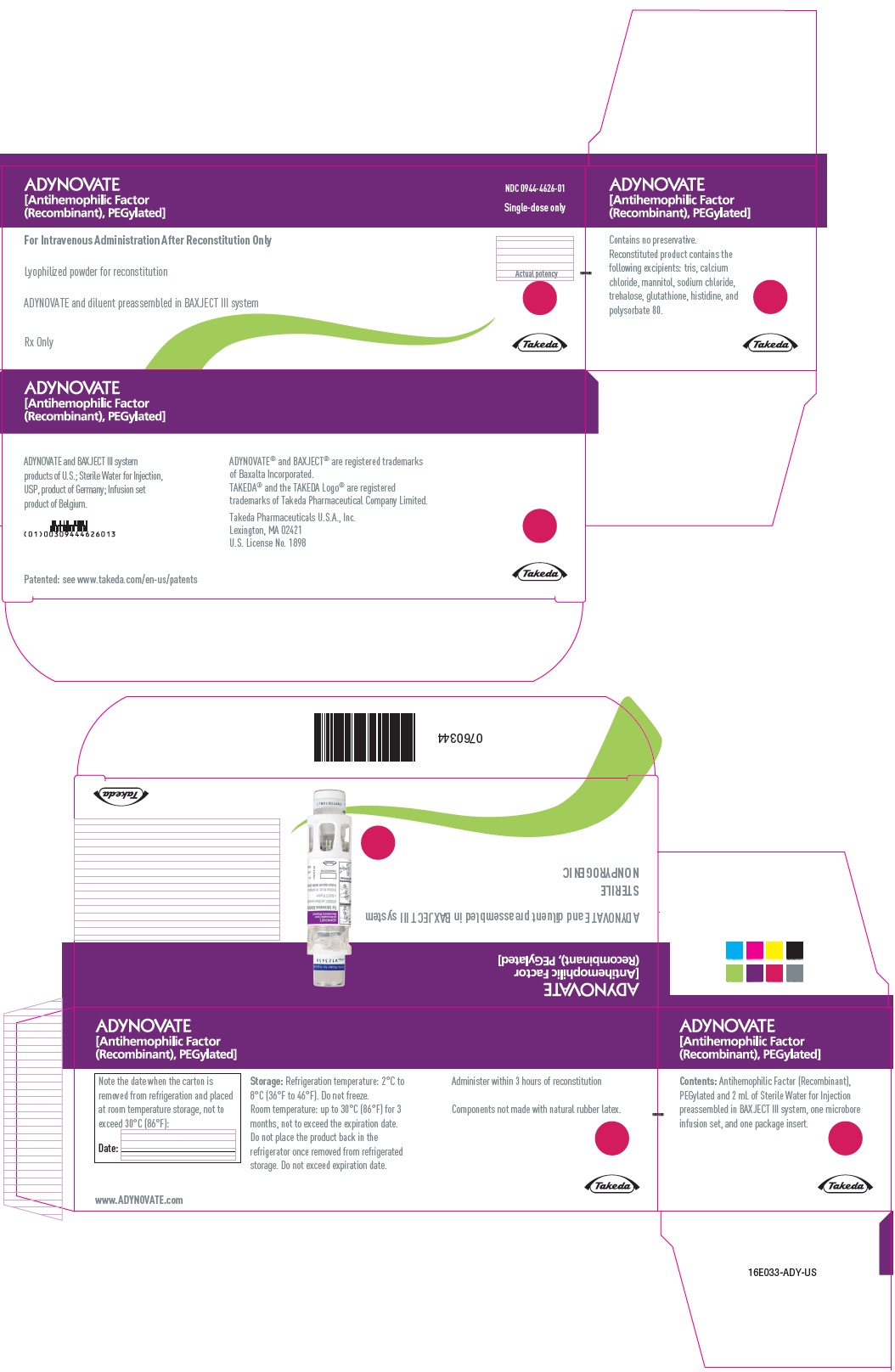
PRINCIPAL DISPLAY PANEL - Kit Carton - 750 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4626-01 - Single-dose only - For Intravenous Administration After Reconstitution Only - Lyophilized powder for ...
-
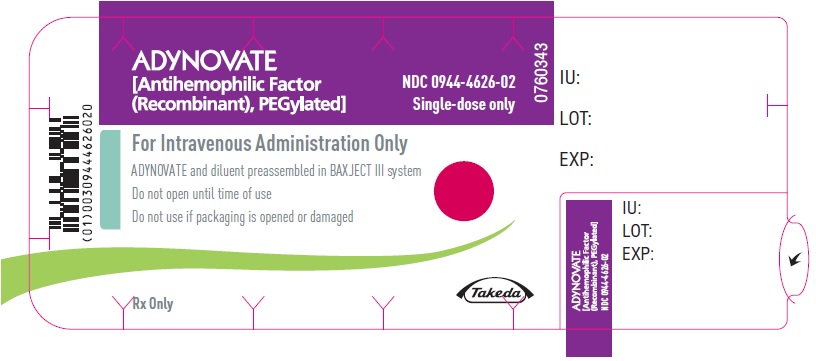
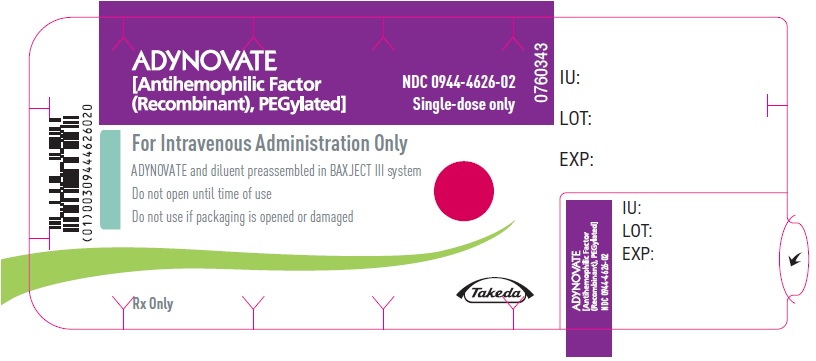
PRINCIPAL DISPLAY PANEL - Barrel Label - 750 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] For Intravenous Administration Only - ADYNOVATE and diluent preassembled - in BAXJECT® III system - Directions for use: see package ...
-
PRINCIPAL DISPLAY PANEL - Blister Label - 750 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4626-02 - Single-dose only - 6521490 - For Intravenous Administration Only - ADYNOVATE and diluent preassembled in BAXJECT ...
-
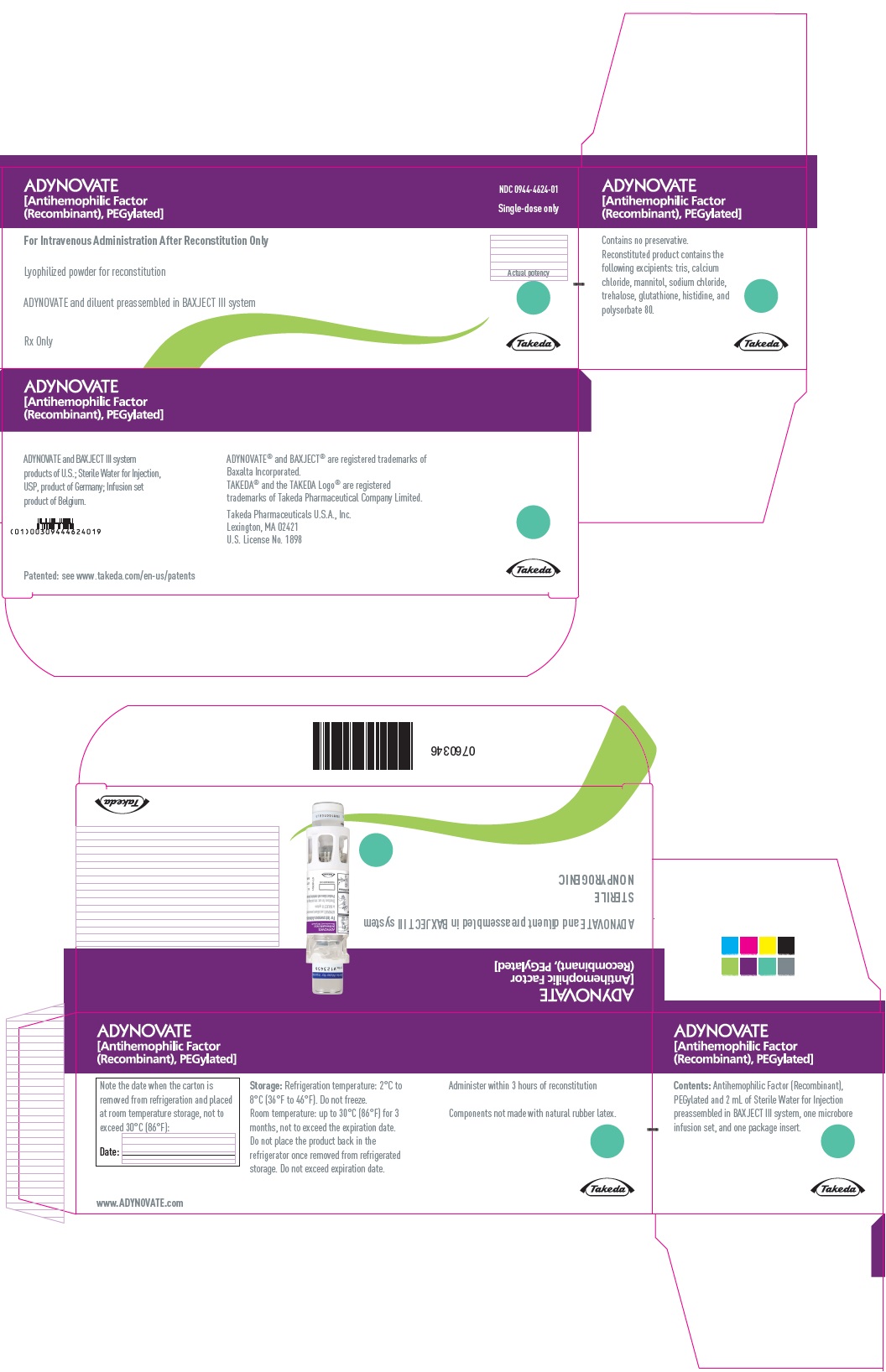
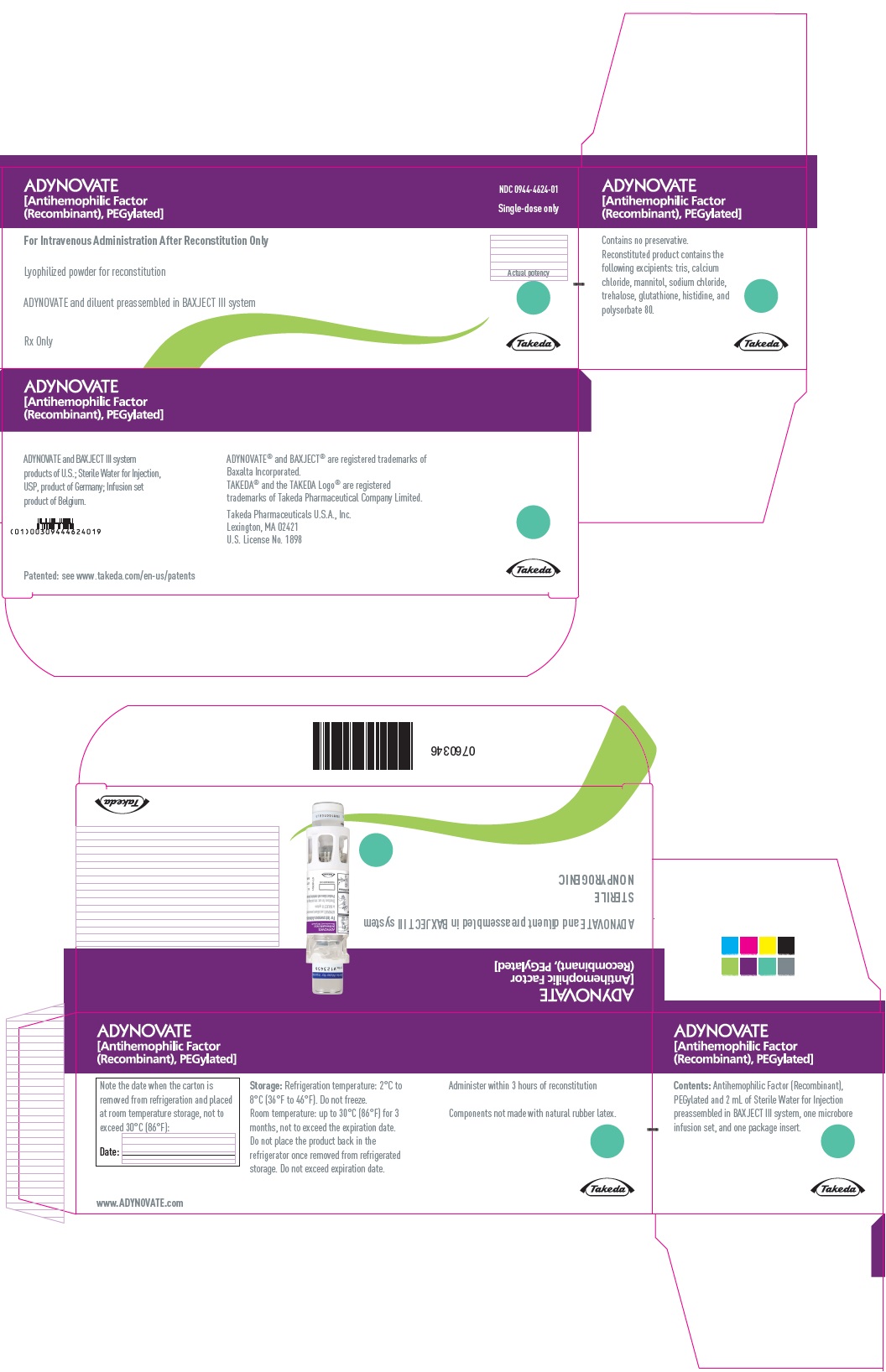
PRINCIPAL DISPLAY PANEL - Kit Carton - 1000 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4624-01 - Single-dose only - For Intravenous Administration After Reconstitution Only - Lyophilized powder for ...
-
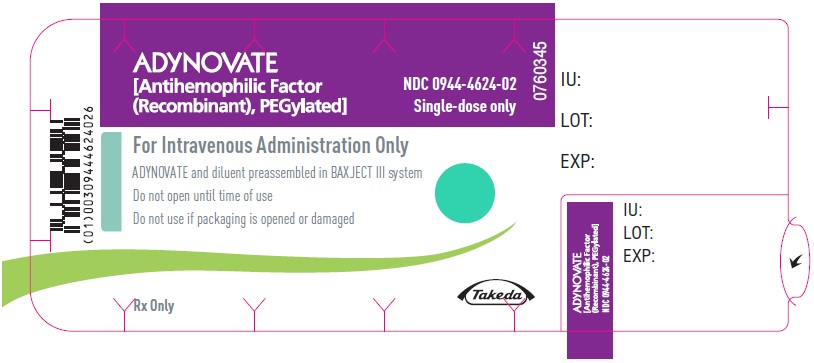
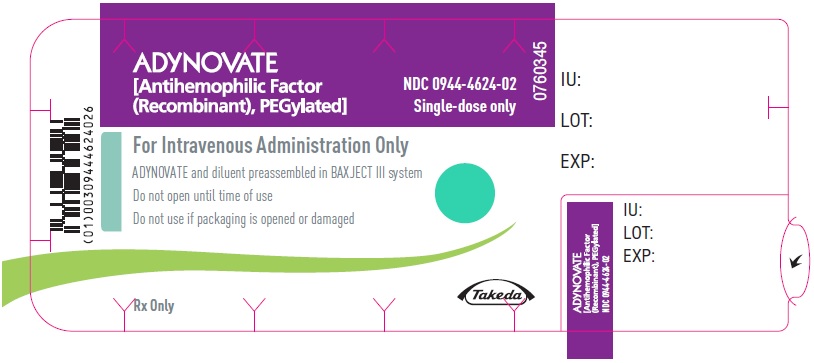
PRINCIPAL DISPLAY PANEL - Barrel Label - 1000 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] For Intravenous Administration Only - ADYNOVATE and diluent preassembled - in BAXJECT® III system - Directions for use: see package ...
-
PRINCIPAL DISPLAY PANEL - Blister Label - 1000 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4624-02 - Single-dose only - 6521493 - For Intravenous Administration Only - ADYNOVATE and diluent preassembled in BAXJECT ...
-
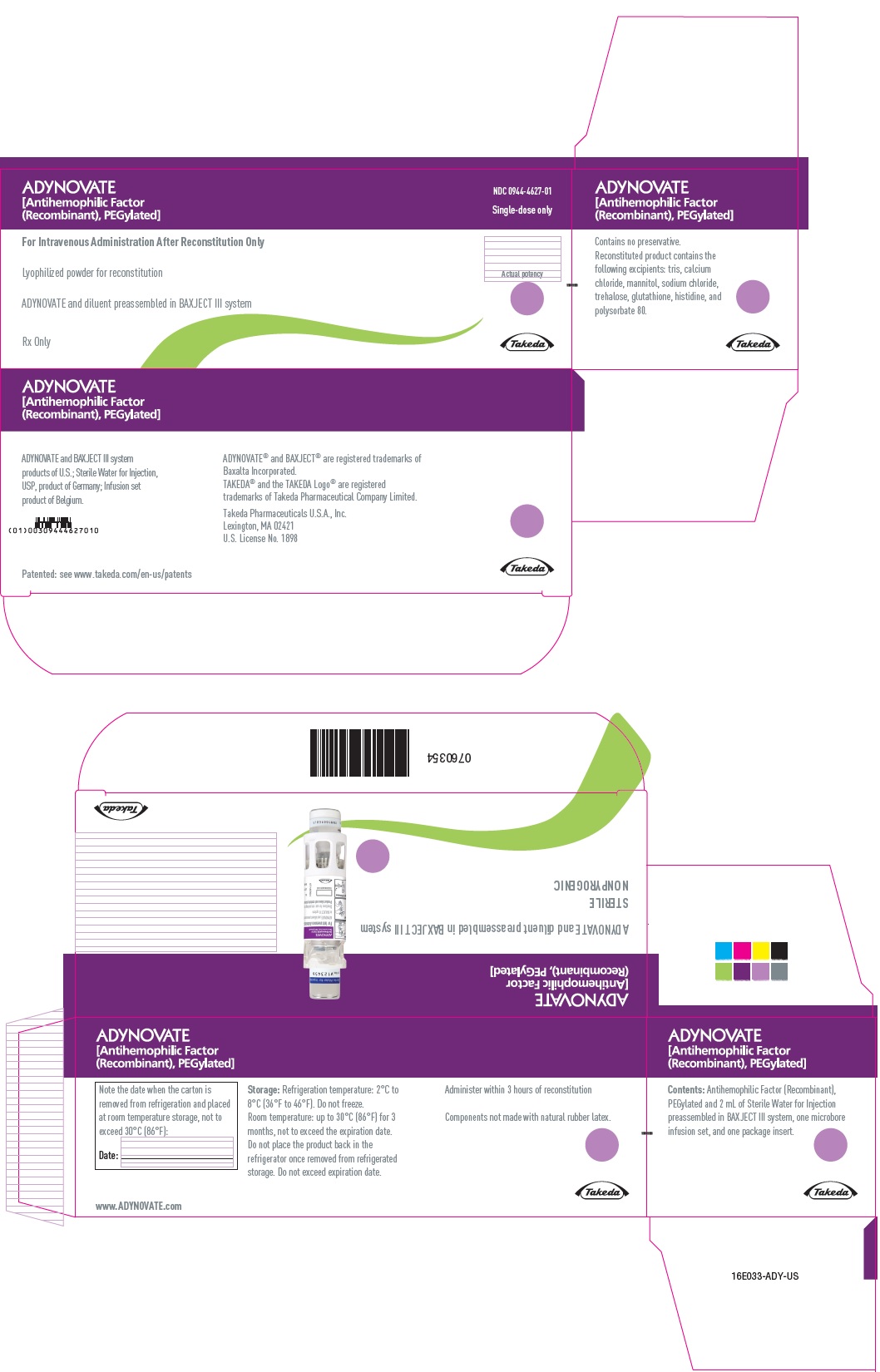
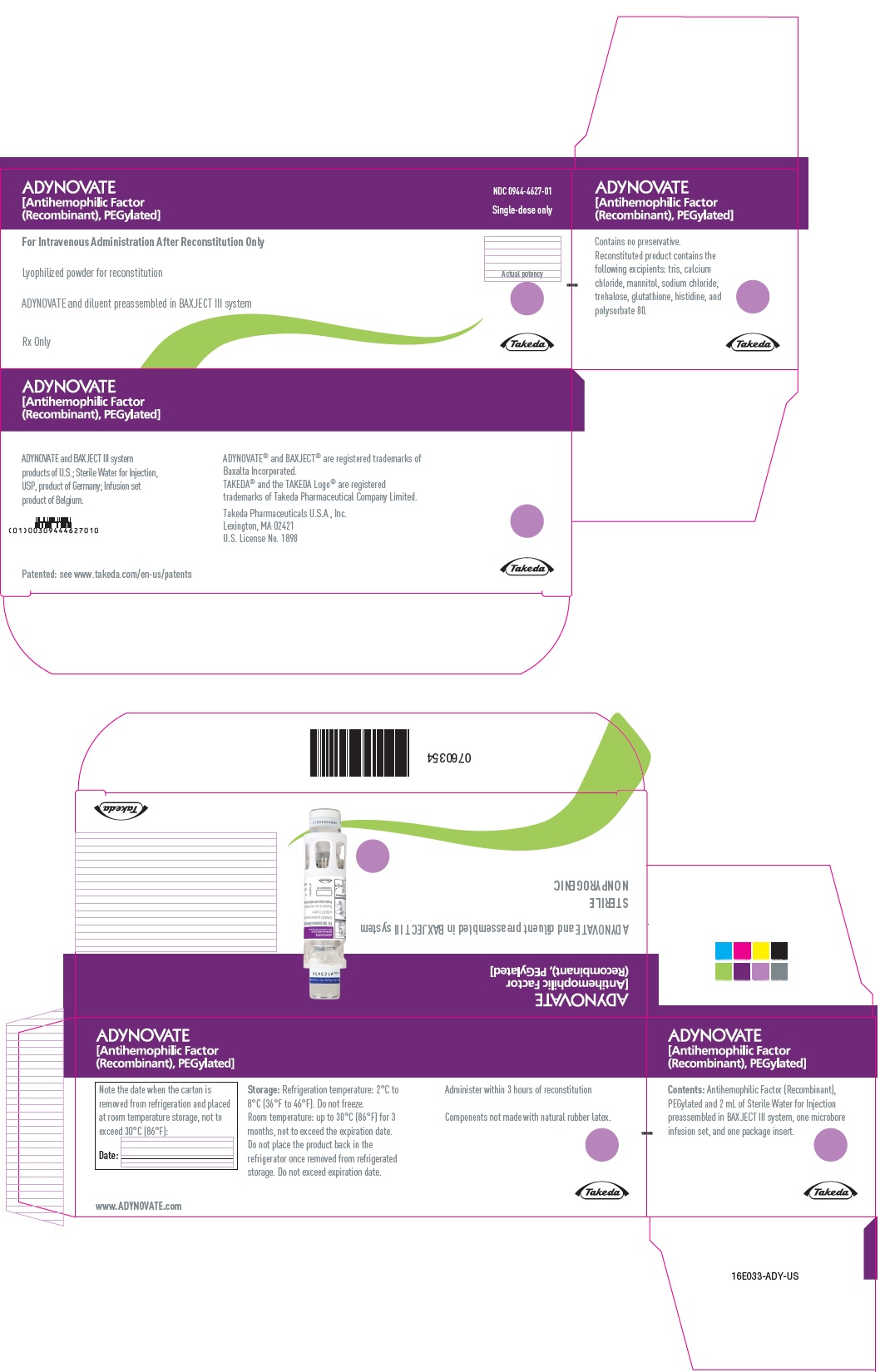
PRINCIPAL DISPLAY PANEL - Kit Carton - 1500 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4627-01 - Single-dose only - For Intravenous Administration After Reconstitution Only - Lyophilized powder for ...
-
PRINCIPAL DISPLAY PANEL - Barrel Label - 1500 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] For Intravenous Administration Only - ADYNOVATE and diluent preassembled - in BAXJECT® III system - Directions for use: see package ...
-
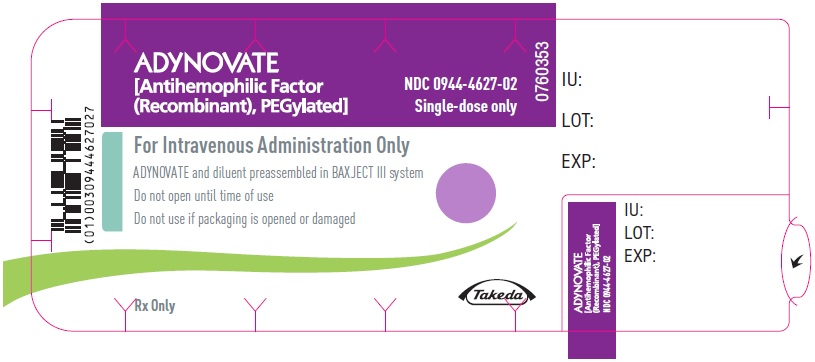
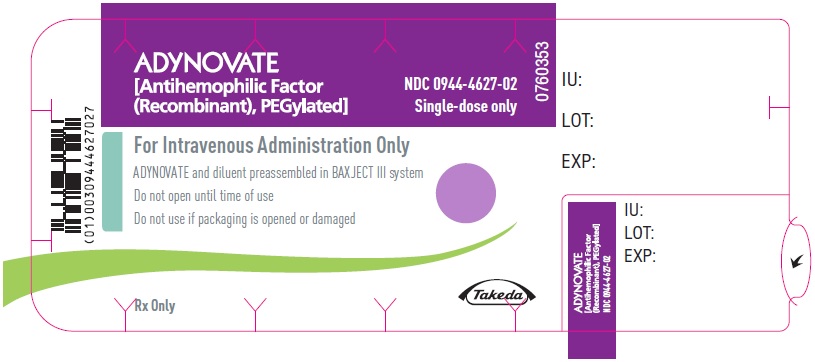
PRINCIPAL DISPLAY PANEL - Blister Label - 1500 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4627-02 - Single-dose only - 6521495 - For Intravenous Administration Only - ADYNOVATE and diluent preassembled in BAXJECT ...
-
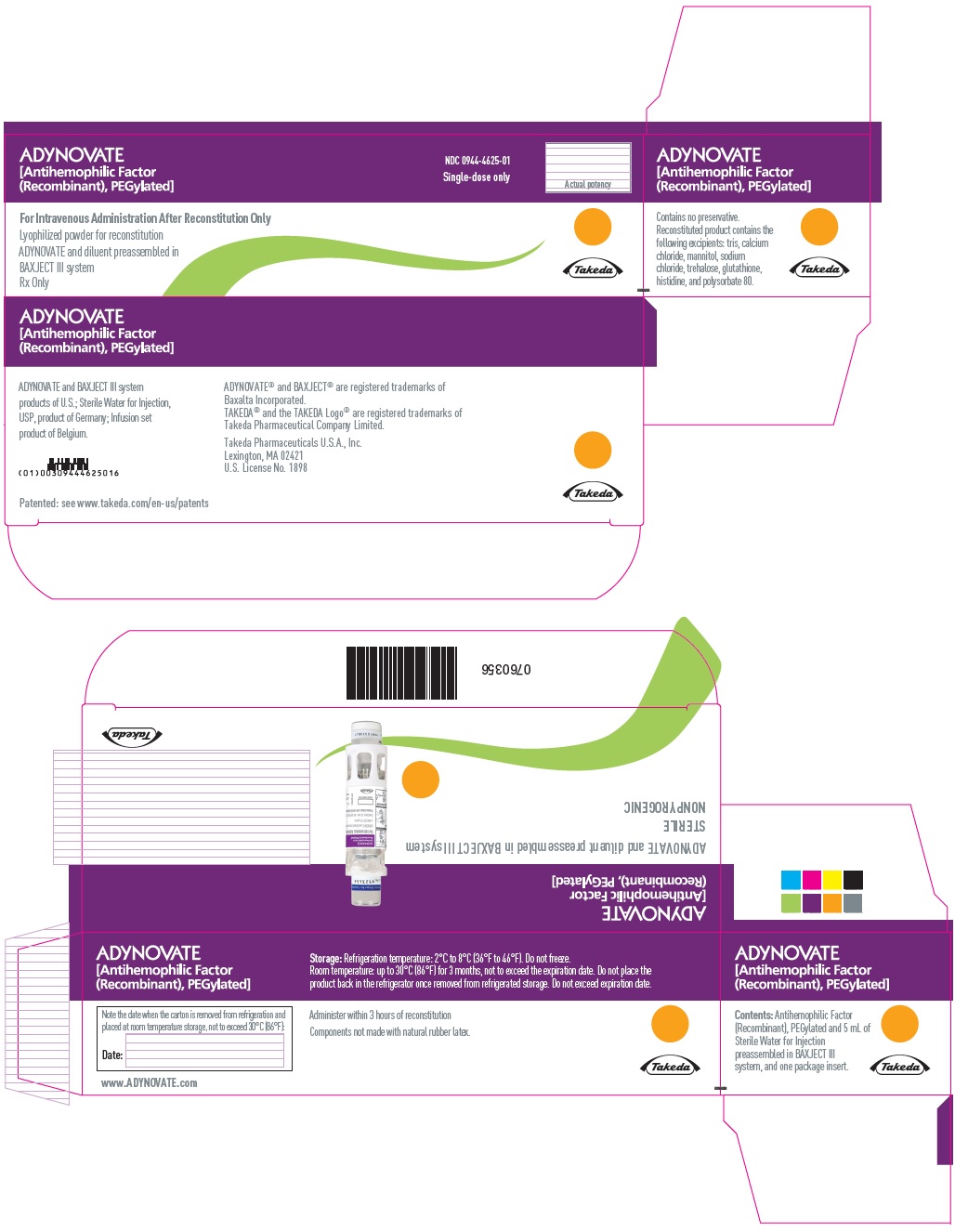
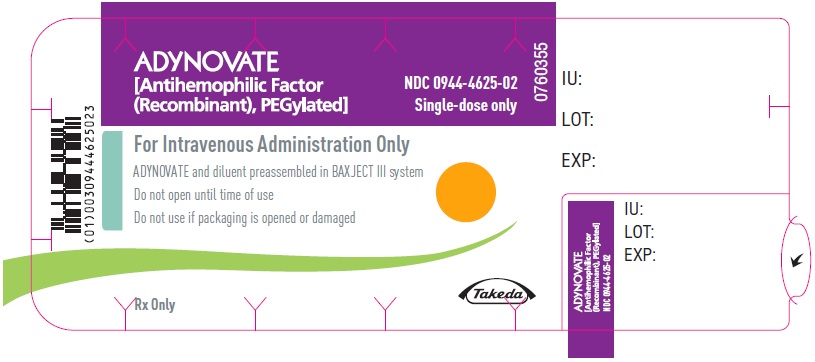
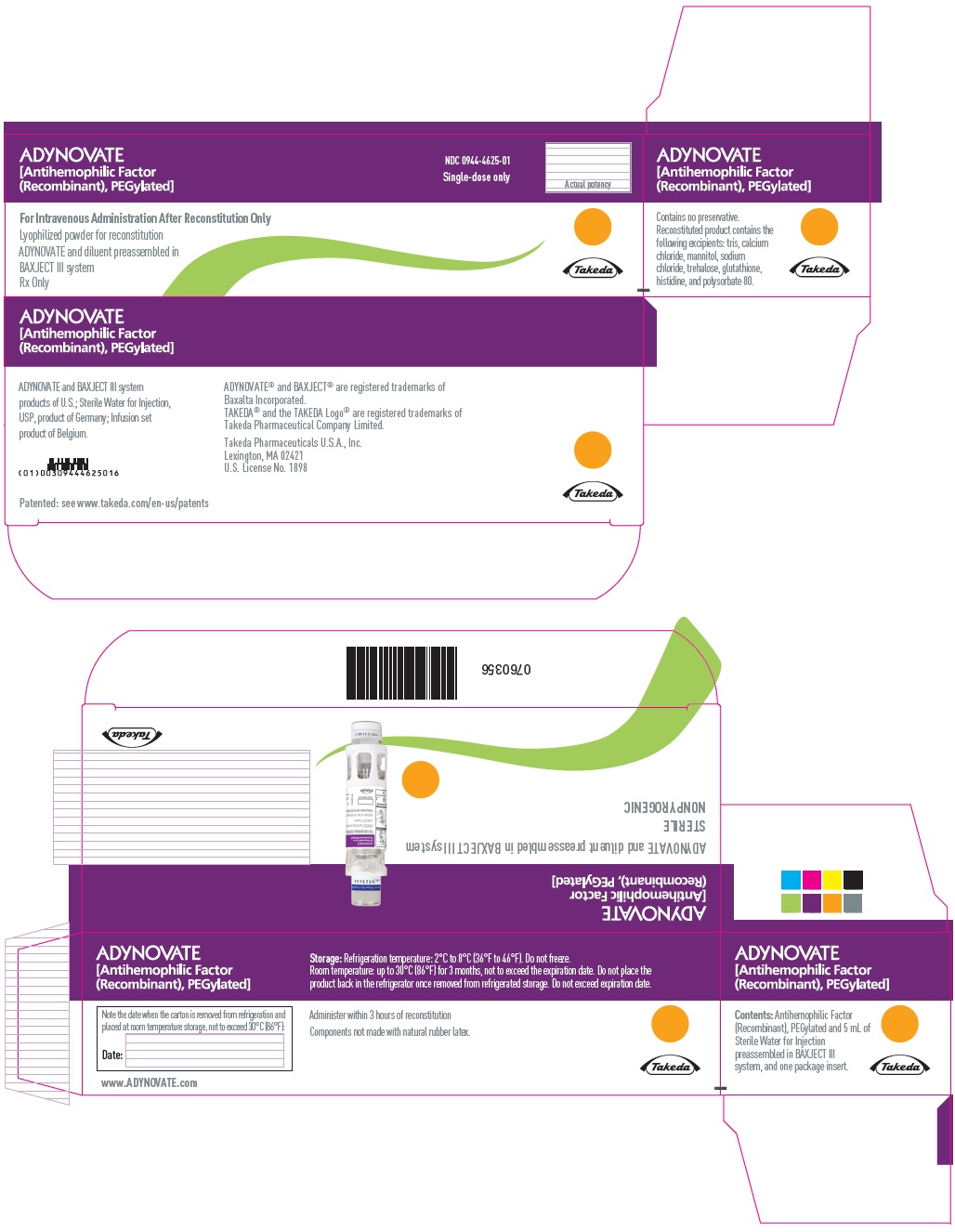
PRINCIPAL DISPLAY PANEL - Kit Carton - 2000 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4625-01 - Single-dose only - For Intravenous Administration After Reconstitution Only - Lyophilized powder for ...
-
PRINCIPAL DISPLAY PANEL - Barrel Label - 2000 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] For Intravenous Administration Only - ADYNOVATE and diluent preassembled - in BAXJECT® III system - Directions for use: see package ...
-
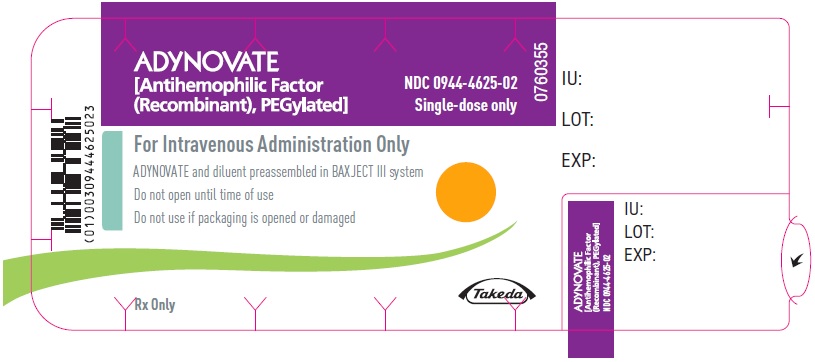
PRINCIPAL DISPLAY PANEL - Blister Label - 2000 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4625-02 - Single-dose only - 6521498 - For Intravenous Administration Only - ADYNOVATE and diluent preassembled in BAXJECT ...
-
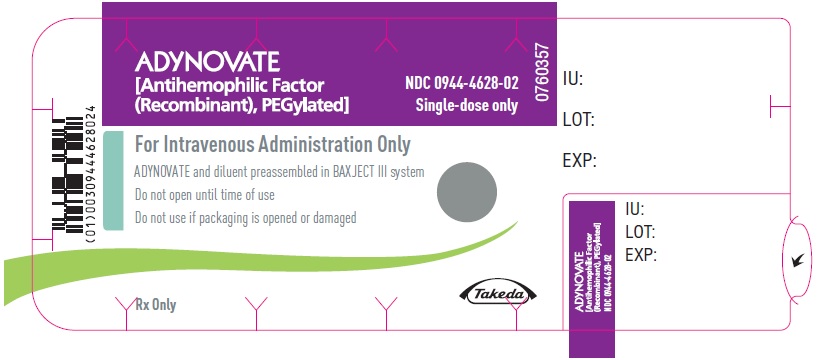
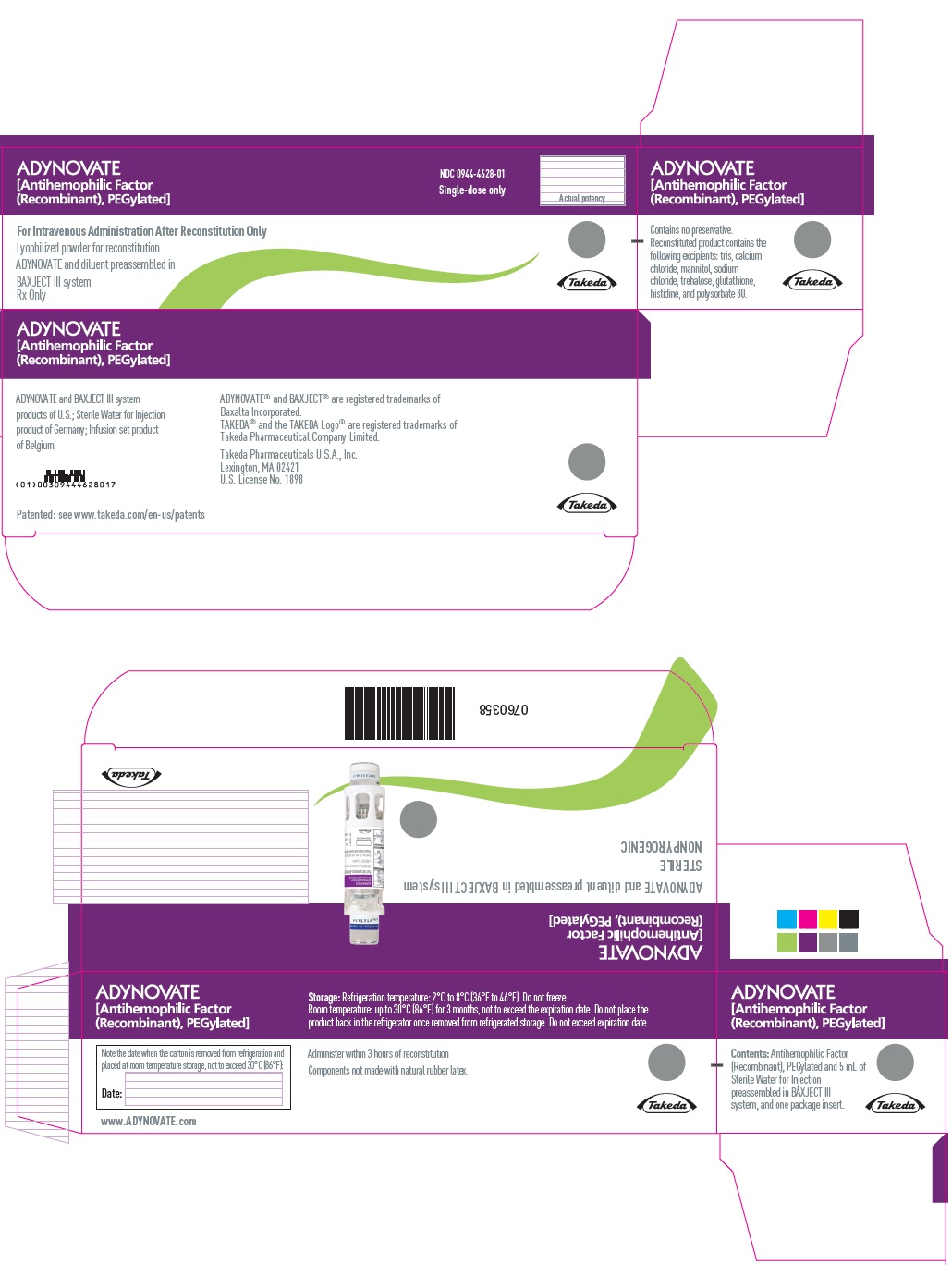
PRINCIPAL DISPLAY PANEL - Kit Carton - 3000 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4628-01 - Single-dose only - For Intravenous Administration After Reconstitution Only - Lyophilized powder for ...
-
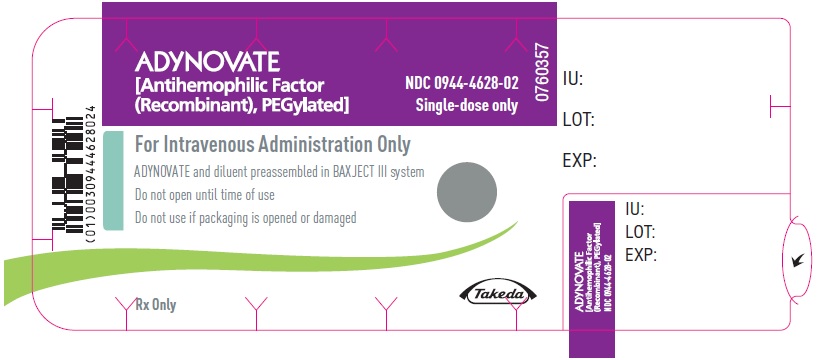
PRINCIPAL DISPLAY PANEL - Barrel Label - 3000 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] For Intravenous Administration Only - ADYNOVATE and diluent preassembled - in BAXJECT® III system - Directions for use: see package ...
-
PRINCIPAL DISPLAY PANEL - Blister Label - 3000 IUADYNOVATE® [Antihemophilic Factor - (Recombinant), PEGylated] NDC 0944-4628-02 - Single-dose only - 6521500 - For Intravenous Administration Only - ADYNOVATE and diluent preassembled in BAXJECT ...
-
PRINCIPAL DISPLAY PANEL - Antihemophilic Factor Vial LabelAntihemophilic Factor (Recombinant), PEGylated - 6521222 - Takeda - Lot No.:
-
PRINCIPAL DISPLAY PANEL - Sterile Water for Injection Vial LabelSterile Water for Injection - Lot No.: Siegfried Hameln GmbH - 0742211
-
INGREDIENTS AND APPEARANCEProduct Information








 are registered trademarks of Takeda Pharmaceutical Company Limited.
are registered trademarks of Takeda Pharmaceutical Company Limited.