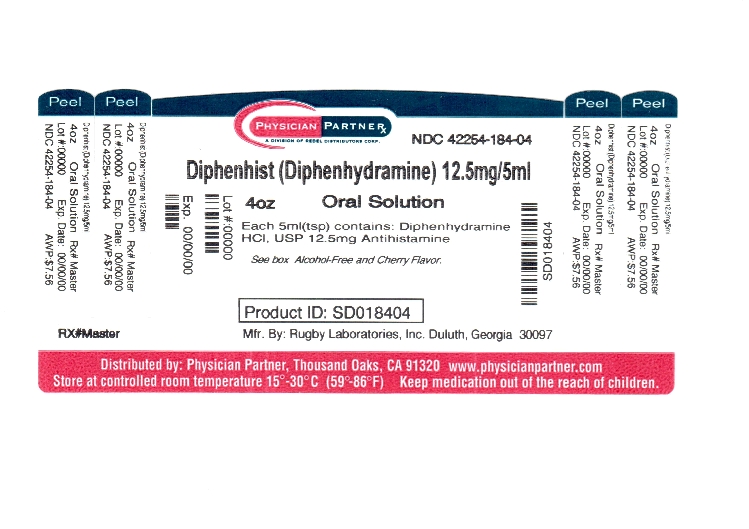
Label: DIPHENHIST- diphenhydramine hydrochloride liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 42254-184-04 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 0536-0770
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 20, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient (in each 5mL teaspoonful)Diphenhydramine HCl USP 12.5 mg
-
PurposeAntihistamine
-
Usestemporarily relieves these symptoms of hay fever or other upper respiratory allergies - runny nose - sneezing - itchy, water eyes - itchy nose or throat
-
WarningsDo not use with any other product containing diphenhydramine, even one used on skin - Ask a doctor before use if you have - glaucoma - trouble urinating due to an enlarged prostate gland - a ...
-
Directionstake every 4 – 6 hours - do not take more than 6 doses in 24 hours - adults and children 12 years and over2 - 4 teaspoonfuls - children 6 to under 12 years1 - 2 teaspoonfuls - children under ...
-
Other informationeach teaspoonful (5 mL) contains: sodium 5 mg - store between 20°-25° C (68°-77°F) protect from light. Store in outer carton until contents used
-
Inactive ingredientsartificial cherry flavor, citric acid, D&C red #33, FD&C red #40, glycerin, polysorbate 20, purified water, saccharin sodium, sodium benzoate, sodium citrate, sorbitol solution
-
Questions or comments?call 1-800-645-2158, 9 am - 5pm ET, Monday - Friday
-
PRINCIPAL DISPLAY PANELCOMPARE TO - ACTIVE INGREDIENT IN - BENADRYL® ALLERGY LIQUID* CHILDREN'S ALCOHOL-FREE - ALLERGY MEDICATION - Diphenhist® ORAL SOLUTION - Diphenydramine HCI, USP - Antihistamine - For Temporary ...
-
INGREDIENTS AND APPEARANCEProduct Information