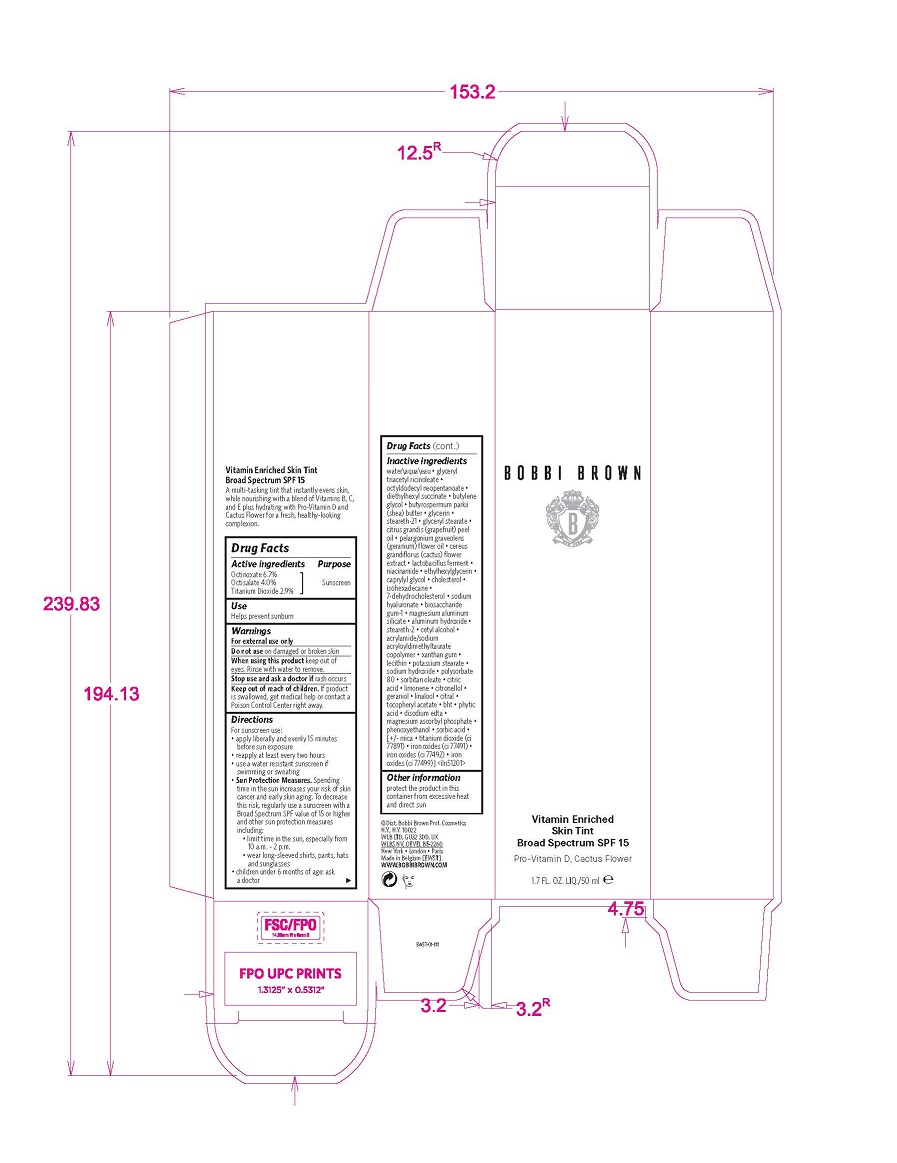
Label: VITAMIN ENRICHED SKIN TINT BROAD SPECTRUM SPF 15 liquid
- NDC Code(s): 64141-033-01, 64141-033-02
- Packager: Bobbi Brown Professional Cosmetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Use
- Warnings
-
Directions
For sunscreen use:
• apply liberally and evenly 15 minutes
before sun exposure
• reapply at least every two hours
• use a water resistant sunscreen if
swimming or sweatingSun Protection Measures. Spending
time in the sun increases your risk of skin
cancer and early skin aging. To decrease
this risk, regularly use a sunscreen with a
Broad Spectrum SPF value of 15 or higher
and other sun protection measures
including:
• limit time in the sun, especially fr om
10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats
and sunglasses
• children under 6 months of age: ask
a doctor -
Inactive ingredients
water\aqua\eau • glyceryl
triacetyl ricinoleate •
octyldodecyl neopentanoate •
diethylhexyl succinate • butylene
glycol • butyrospermum parkii
(shea) butter • glycerin •
steareth-21 • glyceryl stearate •
citrus grandis (grapefruit) peel
oil • pelargonium graveolens
(geranium) flower oil • cereus
grandiflorus (cactus) flower
extract • lactobacillus ferment •
niacinamide • ethylhexylglycerin •
caprylyl glycol • cholesterol •
isohexadecane •
7-dehydrocholesterol • sodium
hyaluronate • biosaccharide
gum-1 • magnesium aluminum
silicate • aluminum hydroxide •
steareth-2 • cetyl alcohol •
acrylamide/sodium
acryloyldimethyltaurate
copolymer • xanthan gum •
lecithin • potassium stearate •
sodium hydroxide • polysorbate
80 • sorbitan oleate • citric
acid • limonene • citronellol •
geraniol • linalool • citral •
tocopheryl acetate • bht • phytic
acid • disodium edta •
magnesium ascorbyl phosphate •
phenoxyethanol • sorbic acid •
[+/- mica • titanium dioxide (ci
77891) • iron oxides (ci 77491) •
iron oxides (ci 77492) • iron
oxides (ci 77499)] - Other Information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITAMIN ENRICHED SKIN TINT BROAD SPECTRUM SPF 15
vitamin enriched skin tint broad spectrum spf 15 liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64141-033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 67 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 40 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 29 mg in 1 mL Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ISOHEXADECANE (UNII: 918X1OUF1E) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM STEARATE (UNII: 17V812XK50) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLYCERYL TRIACETYL RICINOLEATE (UNII: 6TX6N2LEXN) SHEA BUTTER (UNII: K49155WL9Y) GLYCERIN (UNII: PDC6A3C0OX) LIMOSILACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) NIACINAMIDE (UNII: 25X51I8RD4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHOLESTEROL (UNII: 97C5T2UQ7J) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DIETHYLHEXYL SUCCINATE (UNII: 69W9UMG3P8) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SELENICEREUS GRANDIFLORUS FLOWER (UNII: II877K4UNR) LIMONENE, (+)- (UNII: GFD7C86Q1W) GERANIOL (UNII: L837108USY) LINALOOL, (+/-)- (UNII: D81QY6I88E) CITRAL (UNII: T7EU0O9VPP) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FYTIC ACID (UNII: 7IGF0S7R8I) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) PHENOXYETHANOL (UNII: HIE492ZZ3T) SORBIC ACID (UNII: X045WJ989B) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL ALCOHOL (UNII: 936JST6JCN) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) 7-DEHYDROCHOLESTEROL (UNII: BK1IU07GKF) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) STEARETH-21 (UNII: 53J3F32P58) CITRUS MAXIMA FRUIT RIND OIL (UNII: 8U3877WD44) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) WATER (UNII: 059QF0KO0R) STEARETH-2 (UNII: V56DFE46J5) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) MICA (UNII: V8A1AW0880) SODIUM ACRYLOYLDIMETHYLTAURATE-ACRYLAMIDE COPOLYMER (1:1; 90000-150000 MPA.S) (UNII: 5F4963KLHS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64141-033-01 1 in 1 CARTON 05/01/2023 1 50 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:64141-033-02 1 in 1 CARTON 05/01/2023 2 7 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/01/2023 Labeler - Bobbi Brown Professional Cosmetics Inc. (627131279) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(64141-033) , label(64141-033) , pack(64141-033)