Label: SPECTRAMAST DC- ceftiofur hydrochloride suspension
- NDC Code(s): 54771-5278-1, 54771-5278-2
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONSPECTRAMAST® DC - brand of ceftiofur hydrochloride sterile suspension - For Intramammary Infusion in Dry Dairy Cattle Only - FOR USE IN ANIMALS ONLY — NOT FOR HUMAN USE
-
CAUTIONFederal law restricts this drug to use by or on the order of a licensed veterinarian. Federal Law prohibits extra-label use of this drug in dry dairy cattle for disease prevention purposes; at ...
-
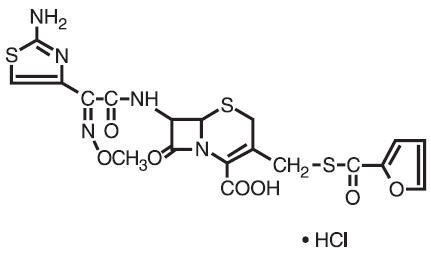
DESCRIPTIONCeftiofur hydrochloride is a cephalosporin antibiotic. Chemical Structure of Ceftiofur Hydrochloride - U-64279A - Chemical Name of Ceftiofur ...
-
INDICATIONS FOR USESPECTRAMAST® DC Ceftiofur Hydrochloride Sterile Suspension is indicated for the treatment of subclinical mastitis in dairy cattle at the time of dry off associated with Staphylococcus aureus ...
-
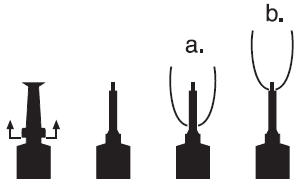
DOSAGEInfuse one (1) syringe into each affected quarter at the time of dry off. DIRECTIONS FOR USING THE PLASTET® DISPOSABLE SYRINGE - The syringe is designed to provide the choice of either ...
-
ADMINISTRATIONTreatment: Wash teats thoroughly with warm water containing a suitable dairy antiseptic. Dry teats thoroughly. Milk out udder completely. Using an appropriate antiseptic teat wipe (containing ...
-
CONTRAINDICATIONSAs with all drugs, the use of SPECTRAMAST® DC Sterile Suspension is contraindicated in animals previously found to be hypersensitive to the drug.
-
SPL UNCLASSIFIED SECTIONDiscard Empty Container: DO NOT REUSE - KEEP OUT OF REACH OF CHILDREN
-
WARNINGSPenicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions ...
-
CONTACT INFORMATIONThe Safety Data Sheet contains more detailed occupational safety information. For a copy of the Safety Data Sheet or to report adverse reactions, call Zoetis Inc. at 1-888-963-8471. For additional ...
-
RESIDUE WARNINGSMilk taken from cows completing a 30-day dry cow period may be used for food with no milk discard due to ceftiofur residues. Following label use, no pre-slaughter withdrawal period is required ...
-
CLINICAL MICROBIOLOGYCeftiofur is a broad-spectrum cephalosporin antibiotic that exerts its effect by inhibiting bacterial cell wall synthesis. Like other ß-lactam antimicrobial agents, the cephalosporins inhibit cell ...
-
EFFECTIVENESSThe effectiveness of a single intramammary (IMM) infusion of ceftiofur hydrochloride for the treatment of subclinical mastitis present at the time of dry off was demonstrated in a randomized block ...
-
ANIMAL SAFETYAn udder irritation study was conducted in 22 healthy lactating dairy cows to assess udder irritation following a single intramammary infusion of a sterile oil-based suspension containing 500 mg ...
-
MILK AND TISSUE RESIDUE DEPLETIONA metabolism study in cattle using radiolabeled ceftiofur provided the data to establish tolerances for ceftiofur-related residues (as desfuroylceftiofur) in tissue and milk. These tolerances of ...
-
STORAGE CONDITIONSStore at controlled room temperature 20° to 25° C (68° to 77° F). Protect from light. Store plastets in carton until used.
-
HOW SUPPLIEDSPECTRAMAST® DC Sterile Suspension is available in cartons containing 1 unbroken package of 12–10 mL PLASTET® Disposable Syringes and in pails containing 12 unbroken packages of 12-10 mL PLASTET ...
-
SPL UNCLASSIFIED SECTIONApproved by FDA under NADA # 141-239 - zoetis - Distributed by: Zoetis Inc. Kalamazoo, MI 49007 - www.spectramast.com or call 1-888-963-8471 - Revised: December 2022 - 40040100
-
PRINCIPAL DISPLAY PANEL - 12 Syringe Carton40040101
-
INGREDIENTS AND APPEARANCEProduct Information