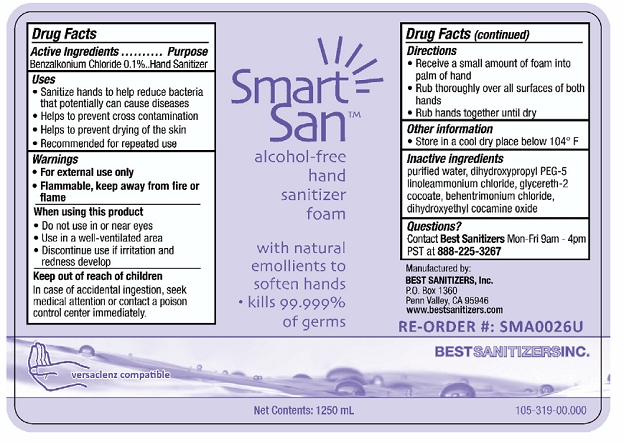
Label: SMART SAN ALCOHOL FREE HAND SANITIZER FOAM- benzalkonium chloride liquid
- NDC Code(s): 59900-118-06, 59900-118-12, 59900-118-48
- Packager: Best Sanitizers, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- When Using this product
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SMART SAN ALCOHOL FREE HAND SANITIZER FOAM
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59900-118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1 g in 1000 mL Inactive Ingredients Ingredient Name Strength GLYCERETH-2 COCOATE (UNII: JWM00VS7HC) WATER (UNII: 059QF0KO0R) DIHYDROXYPROPYL PEG-5 LINOLEAMMONIUM CHLORIDE (UNII: 0Y0NQR2GH1) BEHENTRIMONIUM CHLORIDE (UNII: X7GNG3S47T) DIHYDROXYETHYL COCAMINE OXIDE (UNII: 8AR51R3BL5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59900-118-06 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/15/2009 2 NDC:59900-118-12 250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/15/2009 3 NDC:59900-118-48 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/15/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/15/2009 Labeler - Best Sanitizers, Inc (957473614) Establishment Name Address ID/FEI Business Operations Best Sanitizers, Inc 627278224 manufacture(59900-118)