Label: IMODIUM MULTI-SYMPTOM RELIEF- loperamide hydrochloride and dimethicone tablet
-
NDC Code(s):
50580-338-06,
50580-338-12,
50580-338-18,
50580-338-30, view more50580-338-42, 50580-338-60, 50580-338-61, 50580-338-64
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
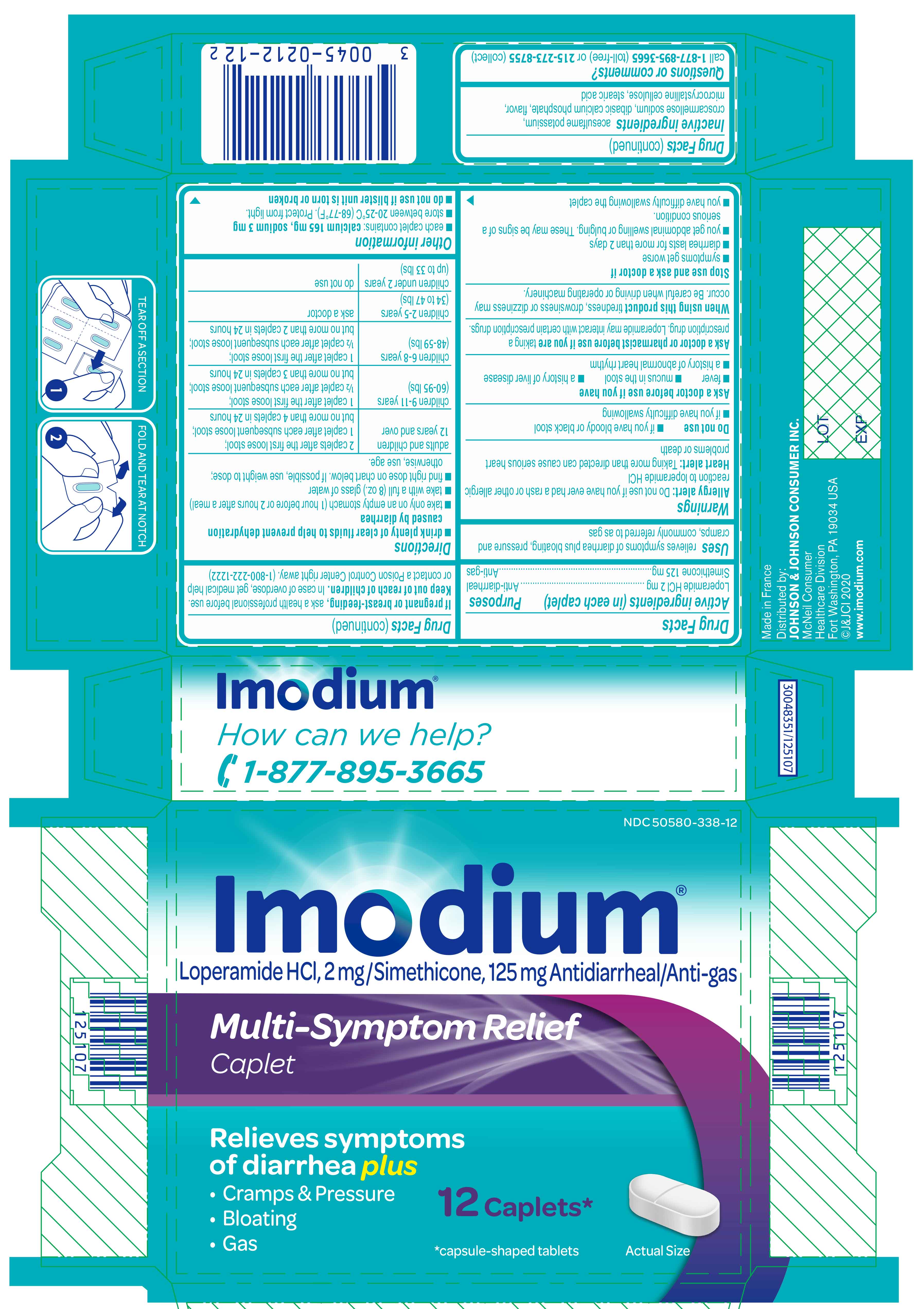
- ACTIVE INGREDIENT
- Uses
-
Warnings
Ask a doctor before use if you have
- fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
-
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- take only on an empty stomach (1 hour before or 2 hours after a meal)
- take with a full (8 oz.) glass of water
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
adults and children 12 years and over 2 caplets after the first loose stool;
1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hourschildren 9-11 years
(60-95 lbs)1 caplet after the first loose stool;
½ caplet after each subsequent loose stool; but no more than 3 caplets in 24 hourschildren 6-8 years
(48-59 lbs)1 caplet after the first loose stool;
½ caplet after each subsequent loose stool; but no more than 2 caplets in 24 hourschildren 2-5 years
(34 to 47 lbs)ask a doctor children under 2 years
(up to 33 lbs)do not use
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IMODIUM MULTI-SYMPTOM RELIEF
loperamide hydrochloride and dimethicone tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50580-338 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 2 mg DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 125 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score 2 pieces Shape OVAL Size 16mm Flavor Imprint Code IMO;2;125 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50580-338-12 2 in 1 CARTON 07/01/2008 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:50580-338-18 3 in 1 CARTON 07/01/2008 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:50580-338-42 1 in 1 CARTON 07/01/2008 09/07/2021 3 42 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:50580-338-30 1 in 1 CARTON 07/01/2008 09/07/2021 4 30 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:50580-338-60 2 in 1 PACKAGE 06/14/2013 07/31/2021 5 1 in 1 CARTON 5 30 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:50580-338-61 4 in 1 CARTON 06/24/2019 6 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 7 NDC:50580-338-64 4 in 1 CARTON 02/26/2024 7 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 8 NDC:50580-338-06 1 in 1 CARTON 03/31/2025 8 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021140 07/01/2008 Labeler - Kenvue Brands LLC (118772437)