Label: ABACAVIR- abacavir sulfate solution
- NDC Code(s): 64980-405-24
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 9, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ABACAVIR ORAL SOLUTION safely and effectively. See full prescribing information for ABACAVIR ORAL SOLUTION. ABACAVIR oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HYPERSENSITIVITY REACTIONS
Serious and sometimes fatal hypersensitivity reactions, with multiple organ involvement, have occurred with abacavir.
Patients who carry the HLA-B*5701 allele are at a higher risk of a hypersensitivity reaction to abacavir; although, hypersensitivity reactions have occurred in patients who do not carry the HLA-B*5701 allele [see Warnings and Precautions (5.1)].
Abacavir is contraindicated in patients with a prior hypersensitivity reaction to abacavir and in HLA-B*5701-positive patients [see Contraindications (4), Warnings and Precautions (5.1)]. All patients should be screened for the HLA-B*5701 allele prior to initiating therapy with abacavir or reinitiation of therapy with abacavir, unless patients have a previously documented HLA-B*5701 allele assessment. Discontinue abacavir immediately if a hypersensitivity reaction is suspected, regardless of HLA-B*5701 status and even when other diagnoses are possible [see Contraindications (4), Warnings and Precautions (5.1)].
Following a hypersensitivity reaction to abacavir, NEVER restart abacavir or any other abacavir-containing product because more severe symptoms, including death can occur within hours. Similar severe reactions have also occurred rarely following the reintroduction of abacavir-containing products in patients who have no history of abacavir hypersensitivity [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEAbacavir oral solution, in combination with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus (HIV-1) infection.
-
2 DOSAGE AND ADMINISTRATION2.1 Screening for HLA-B*5701 Allele prior to Starting Abacavir Oral Solution - Screen for the HLA-B*5701 allele prior to initiating therapy with abacavir oral solution [see Boxed Warning ...
-
3 DOSAGE FORMS AND STRENGTHSAbacavir oral solution USP, each mL containing abacavir sulfate USP equivalent to 20 mg of abacavir, is a clear to opalescent, yellowish, strawberry-banana-flavored liquid.
-
4 CONTRAINDICATIONSAbacavir oral solution is contraindicated in patients: who have the HLA-B*5701 allele [see Warnings and Precautions (5.1)]. with prior hypersensitivity reaction to abacavir [see Warnings and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Serious and sometimes fatal hypersensitivity reactions have occurred with abacavir. These hypersensitivity reactions have included multi-organ failure and ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in other sections of the labeling: Serious and sometimes fatal hypersensitivity reactions [see Boxed Warning, Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Methadone - In a trial of 11 HIV-1-infected subjects receiving methadone-maintenance therapy with 600 mg of abacavir twice daily (twice the currently recommended dose), oral methadone ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to abacavir during pregnancy. Healthcare Providers ...
-
10 OVERDOSAGEThere is no known specific treatment for overdose with abacavir. If overdose occurs, the patient should be monitored and standard supportive treatment applied as required. It is not known whether ...
-
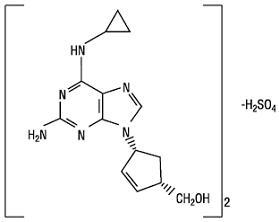
11 DESCRIPTIONAbacavir sulfate is a synthetic carbocyclic nucleoside analogue with inhibitory activity against HIV-1. The chemical name of abacavir sulfate is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Abacavir is an antiretroviral agent [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Pharmacokinetics in Adults - The pharmacokinetic properties of abacavir were ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity - Abacavir was administered orally at 3 dosage levels to separate groups of mice and rats in 2-year carcinogenicity ...
-
14 CLINICAL STUDIES14.1 Adult Trials - Therapy-Naive Adults - CNA30024 was a multicenter, double-blind, controlled trial in which 649 HIV-1-infected, therapy-naive adults were randomized and received either ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAbacavir Oral Solution USP: It is a clear to opalescent, yellowish, strawberry-banana-flavored liquid. Each mL of the solution contains abacavir sulfate USP equivalent to 20 mg of abacavir. It is ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Hypersensitivity Reactions - Inform patients: that a Medication Guide and Warning Card summarizing the ...
-
MEDICATION GUIDEAbacavir Oral Solution USP - (a bak' a vir) What is the most important information I should know about abacavir oral solution? Abacavir oral solution can cause serious side effects ...
-
SPL UNCLASSIFIED SECTION(Front of Card) WARNING CARD - Abacavir Oral Solution USP - Patients taking abacavir oral solution may have a serious allergic reaction (hypersensitivity reaction) that can cause death. If you ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg/mL (240 mL Bottle Label)Rising® NDC 64980-405-24 - PHARMACEUTICALS - Abacavir - Oral Solution USP - 20 mg/mL - Notice to Authorized Dispenser: Each time ABACAVIR ORAL ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg/mL (240 mL Carton Label)Rising® NDC 64980-405-24 - PHARMACEUTICALS - Abacavir - Oral Solution USP - 20 mg/mL - Notice to Authorized Dispenser: Each time ABACAVIR ORAL SOLUTION - USP is ...
-
INGREDIENTS AND APPEARANCEProduct Information