Label: METHYLPHENIDATE HYDROCHLORIDE tablet
-
NDC Code(s):
43386-573-01,
43386-573-03,
43386-573-05,
43386-574-01, view more43386-574-03, 43386-574-05, 43386-575-01, 43386-575-03, 43386-575-05
- Packager: Lupin Pharmaceuticals,Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use
METHYLPHENIDATE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for METHYLPHENIDATE HYDROCHLORIDE TABLETS.
METHYLPHENIDATE HYDROCHLORIDE tablets, for oral use, CII
Initial U.S. Approval: 1955WARNING: ABUSE, MISUSE, AND ADDICTION
See full prescribing information for complete boxed warning.
Methylphenidate hydrochloride tablets has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including methylphenidate hydrochloride tablets, can result in overdose and death (5.1, 9.2, 10):
- Before prescribing methylphenidate hydrochloride tablets, assess each patient's risk for abuse, misuse, and addiction.
- Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug.
- Throughout treatment, reassess each patient's risk and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Methylphenidate Hydrochloride is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorders (ADHD) and Narcolepsy (1).
DOSAGE AND ADMINISTRATION
Methylphenidate Hydrochloride Tablets (2.2): (2)
- Pediatric Patients 6 Years and Older: Start with 5 mg twice daily (before breakfast and lunch), titrating the dose weekly in 5- to 10 - mg increments. Dosages above 60 mg/day are not recommended.
- Adults: Average daily dosage is 20 mg to 30 mg, administered 2 or 3 times daily, preferably 30 to 45 minutes before meals. Maximum total daily dosage is 60 mg.
DOSAGE FORMS AND STRENGTHS
- Tablets: 5 mg, 10 mg, and 20 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risks to Patients with Serious Cardiac Disease: Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmias, coronary artery disease, or other serious cardiac disease (5.2).
- Increased Blood Pressure and Heart Rate: Monitor blood pressure and pulse (5.3).
- Psychiatric Adverse Reactions: Prior to initiating methylphenidate hydrochloride tablets, screen patients for risk factors for developing a manic episode. If new psychotic or manic symptoms occur, consider discontinuing methylphenidate hydrochloride tablets (5.4).
- Priapism: If abnormally sustained or frequent and painful erections occur, patients should seek immediate medical attention (5.5).
- Peripheral Vasculopathy, Including Raynaud's Phenomenon: Careful observation for digital changes is necessary during methylphenidate hydrochloride tablets treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for patients who develop signs or symptoms of peripheral vasculopathy (5.6).
- Long-Term Suppression of Growth in Pediatric Patients: Closely monitor growth (height and weight) in pediatric patients. Pediatric patients not growing or gaining height or weight as expected may need to have their treatment interrupted (5.7).
- Acute Angle Closure Glaucoma: methylphenidate hydrochloride tablets -treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist (5.8).
- Increased Intraocular Pressure (IOP) and Glaucoma: Prescribe methylphenidate hydrochloride tablets to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor patients with a history of increased IOP or open angle glaucoma (5.9).
- Motor and Verbal Tics, and Worsening of Tourette's Syndrome: Before initiating methylphenidate hydrochloride tablets, assess the family history and clinically evaluate patients for tics or Tourette's syndrome. Regularly monitor patients for the emergence or worsening of tics or Tourette's syndrome. Discontinue
ADVERSE REACTIONS
Common adverse reactions: tachycardia, palpitations, headache, insomnia, anxiety, hyperhidrosis, weight loss, decreased appetite, dry mouth, nausea, and abdominal pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 866-403-7592 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
- Antihypertensive Drugs: Monitor blood pressure. Adjust dosage of antihypertensive drug as needed (7.1).
See 17 for PATIENT COUNSELING INFORMATION and
Medication Guide.
See 17 for Medication Guide.
Revised: 3/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ABUSE AND DEPENDENCE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Pretreatment Screening
2.2 General Dosing Information
2.3 Dose Reduction and Discontinuation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Abuse, Misuse, and Addiction
5.2 Risks to Patients with Serious Cardiac Disease
5.3 Increased Blood Pressure and Heart Rate
5.4 Psychiatric Adverse Reactions
5.5 Priapism
5.6 Peripheral Vasculopathy, Including Raynaud’s Phenomenon
5.7 Long-Term Suppression of Growth in Pediatric Patients
5.8 Acute Angle Closure Glaucoma
5.9 Increased Intraocular Pressure and Glaucoma
5.10 Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Clinically Important Drug Interactions with Methylphenidate Hydrochloride tablets
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ABUSE AND DEPENDENCE
WARNING: ABUSE, MISUSE, AND ADDICTION
Methylphenidate hydrochloride tablets has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including methylphenidate hydrochloride tablets, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing methylphenidate hydrochloride tablets, assess each patient's risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout methylphenidate hydrochloride tablets treatment, reassess each patient's risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction [see Warnings and Precautions (5.1) and Drug Abuse and Dependence (9.2)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Pretreatment Screening
• for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions (5.2)].
• the family history and clinically evaluate patients for motor or verbal tics or Tourette's syndrome before initiating methylphenidate hydrochloride tablets [see Warnings and Precautions (5.10)].
2.2 General Dosing Information
Methylphenidate Hydrochloride Tablets
Pediatric Patients 6 years and Older: Start with 5 mg orally twice daily (before breakfast and lunch). Increase dosage gradually, in increments of 5-to10- mg weekly. Daily dosage above 60 mg is not recommended.
Adults: Average dosage is 20 to 30 mg daily. Administer orally in divided doses 2 or 3 times daily, preferably 30 to 45 minutes before meals. Maximum total daily dosage is 60 mg. Patients who are unable to sleep if medication is taken late in the day should take the last dose before 6 p.m.
2.3 Dose Reduction and Discontinuation
If paradoxical worsening of symptoms or other adverse reactions occur, reduce the dosage, or, if necessary, discontinue methylphenidate hydrochloride. If improvement is not observed after appropriate dosage adjustment over a one-month period, the drug should be discontinued.
-
3 DOSAGE FORMS AND STRENGTHS
- 5 mg, light yellow colored, round, flat face tablets with "n above 573" debossed on one side and plain on the other side.
- 10 mg, white to off white colored, round, flat face tablets with "n above 574" debossed on one side and bisected line debossed on the other side
- 20 mg, light yellow colored, round biconvex tablets with "n above 575" debossed on one side and bisect line debossed on the other side
-
4 CONTRAINDICATIONS
- Hypersensitivity to methylphenidate or other components of methylphenidate hydrochloride tablets. Hypersensitivity reactions, such as angioedema and anaphylactic reactions, have been reported in patients treated with methylphenidate [see Adverse Reactions (6.1) ].
- Concomitant treatment with monoamine oxidase inhibitors (MAOIs), or within 14 days following discontinuation of treatment with an MAOI, because of the risk of hypertensive crises [see Drug Interactions (7.1) ].
-
5 WARNINGS AND PRECAUTIONS
5.1 Abuse, Misuse, and Addiction
Methylphenidate hydrochloride tablets have a high potential for abuse and misuse. The use of methylphenidate hydrochloride tablets exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Methylphenidate hydrochloride tablets can be diverted for non-medical use into illicit channels or distribution [see Drug Abuse and Dependence (9.2)]. Misuse and abuse of CNS stimulants, including methylphenidate hydrochloride tablets, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing methylphenidate hydrochloride tablets, assess each patient's risk for abuse, misuse, and addiction. Educate patients and their families about these risks and proper disposal of any unused drug. Advise patients to store methylphenidate hydrochloride tablets in a safe place, preferably locked, and instruct patients to not give methylphenidate hydrochloride tablets to anyone else. Throughout methylphenidate hydrochloride tablets treatment, reassess each patient's risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
5.2 Risks to Patients with Serious Cardiac Disease
Sudden death has been reported in patients with structural cardiac abnormalities or other serious cardiac disease who are treated with CNS stimulants at the recommended ADHD dosage.
Avoid methylphenidate hydrochloride tablets use in patients with known serious structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmia, coronary artery disease, or other serious cardiac disease.
5.3 Increased Blood Pressure and Heart Rate
CNS stimulants cause an increase in blood pressure (mean increase approximately 2 to 4 mmHg) and heart rate (mean increase approximately 3 to 6 beats per minute). Individuals may have larger increases. Monitor all patients for hypertension and tachycardia.
5.4 Psychiatric Adverse Reactions
Exacerbation of Preexisting Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a preexisting psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar Disorder
CNS stimulants may induce a manic or mixed mood episode in patients. Prior to initiating treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, or depression).
New Psychotic or Manic Symptoms
CNS stimulants, at recommended doses, may cause psychotic or manic symptoms (e.g., hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania. If such symptoms occur, consider discontinuing methylphenidate hydrochloride. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients, compared to 0 in placebo-treated patients. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients, compared to 0% of placebo-treated patients. If such symptoms occur, consider discontinuing methylphenidate hydrochloride tablets
5.5 Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate use in both adult and pediatric male patients. Although priapism was not reported with methylphenidate initiation, it developed after some time on methylphenidate, often subsequent to an increase in dosage. Priapism has also occurred during methylphenidate withdrawal (drug holidays or during discontinuation). Methylphenidate hydrochloride tablets-treated patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
5.6 Peripheral Vasculopathy, Including Raynaud’s Phenomenon
CNS stimulants, including methylphenidate hydrochloride, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in postmarketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
5.7 Long-Term Suppression of Growth in Pediatric Patients
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in pediatric patients ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated patients over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated pediatric patients (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this development period.
Closely monitor growth (weight and height) in pediatric patients treated with CNS stimulants, including methylphenidate hydrochloride. Patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
5.8 Acute Angle Closure Glaucoma
There have been reports of angle closure glaucoma associated with methylphenidate treatment.
Although the mechanism is not clear, methylphenidate hydrochloride tablets -treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist.
5.9 Increased Intraocular Pressure and Glaucoma
There have been reports of an elevation of intraocular pressure (IOP) associated with methylphenidate treatment [see Adverse Reactions (6.2)].
Prescribe methylphenidate hydrochloride tablets and methylphenidate to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor methylphenidate hydrochloride tablets -treated patients with a history of abnormally increased IOP or open angle glaucoma.
5.10 Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
CNS stimulants, including methylphenidate, have been associated with the onset or exacerbation of motor and verbal tics. Worsening of Tourette's syndrome has also been reported [see Adverse Reactions (6.2)].
Before initiating methylphenidate hydrochloride tablets, assess the family history and clinically evaluate patients for tics or Tourette's syndrome. Regularly monitor methylphenidate hydrochloride tablets -treated patients for the emergence or worsening of tics or Tourette's syndrome, and discontinue treatment if clinically appropriate.
-
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Abuse, Misuse, and Addiction [see Boxed Warning, Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2, 9.3)]
- Known hypersensitivity to methylphenidate or other ingredients of methylphenidate hydrochloride tablets[see Contraindications (4)]
- Hypertensive crisis with Concomitant Use of Monoamine Oxidase Inhibitors [see Contraindications (4), Drug Interactions (7.1)]
- Risks to Patients with Serious Cardiac Disease [see Warnings and Precautions (5.2)]
- Increased Blood Pressure and Heart Rate [see Warnings and Precautions (5.3)]
- Psychiatric Adverse Reactions [see Warnings and Precautions (5.4)]
- Priapism [see Warnings and Precautions (5.5)]
- Peripheral Vasculopathy, Including Raynaud's Phenomenon [see Warnings and Precautions (5.6)]
- Long-Term Suppression of Growth in Pediatric Patients [see Warnings and Precautions (5.7)]
- Acute Angle Closure Glaucoma [see Warnings and Precautions (5.8)]
- Increased Intraocular Pressure and Glaucoma [see Warnings and Precautions (5.9)]
- Motor and Verbal Tics, and Worsening of Tourette's Syndrome [see Warnings and Precautions (5.10)]
The following adverse reactions associated with the use of all methylphenidate hydrochloride tablets, and other methylphenidate products were identified in clinical trials, spontaneous reports, and literature. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Adverse Reactions Reported with Methylphenidate Hydrochloride
Infections and Infestations: nasopharyngitis
Blood and the Lymphatic System Disorders: leukopenia, thrombocytopenia, anemia
Immune System Disorders: hypersensitivity reactions, including angioedema, and anaphylaxis
Metabolism and Nutrition Disorders: decreased appetite, reduced weight gain, and suppression of growth during prolonged use in pediatric patients
Psychiatric Disorders: insomnia, anxiety, restlessness, agitation, psychosis (sometimes with visual and tactile hallucinations), depressed mood, depression
Nervous System Disorders: headache, dizziness, tremor, dyskinesia, including choreoatheetoid movements, drowsiness, convulsions, cerebrovascular disorders (including vasculitis, cerebral hemorrhages and cerebrovascular accidents), serotonin syndrome in combination with serotonergic drugs
Eye Disorders: blurred vision, difficulties in visual accommodation
Cardiac Disorders: tachycardia, palpitations, increased blood pressure, arrhythmias, angina pectoris
Respiratory, Thoracic, and Mediastinal Disorders: cough
Gastrointestinal Disorders: dry mouth, nausea, vomiting, abdominal pain, dyspepsia
Hepatobiliary Disorders: abnormal liver function, ranging from transaminase elevation to severe hepatic injury
Skin and Subcutaneous Tissue Disorders: hyperhidrosis, pruritus, urticaria, exfoliative dermatitis, scalp hair loss, erythema multiforme rash, thrombocytopenic purpura
Musculoskeletal and Connective Tissue Disorders: arthralgia, muscle cramps, rhabdomyolysis
Investigations: weight loss (adult ADHD patients)
Vascular Disorders: peripheral coldness, Raynaud's phenomenon
Additional Adverse Reactions Reported with Other Methylphenidate-Containing Products
The list below shows adverse reactions not listed for methylphenidate hydrochloride tablets that have been reported with other methylphenidate-containing products.
Blood and Lymphatic Disorders: pancytopenia
Immune System Disorders: hypersensitivity reactions, such as auricular swelling, bullous conditions, eruptions, exanthemas
Psychiatric Disorders: affect lability, mania, disorientation, and libido changes
Nervous System Disorders: migraine, motor and verbal tics
Eye Disorders: diplopia, increased intraocular pressure mydriasis
Cardiac Disorders: sudden cardiac death, myocardial infarction, bradycardia, extrasystole
Respiratory, Thoracic, and Mediastinal Disorders: pharyngolaryngeal pain, dyspnea
Gastrointestinal Disorders: diarrhea, constipation
Skin and Subcutaneous Tissue Disorders: angioneurotic edema, erythema, fixed drug eruption
Musculoskeletal, Connective Tissue, and Bone Disorders: myalgia, muscle twitching
Renal and Urinary Disorders: hematuria
Reproductive System and Breast Disorders: gynecomastia
General Disorders: fatigue, hyperpyrexia
Urogenital Disorders: priapism
-
7 DRUG INTERACTIONS
7.1 Clinically Important Drug Interactions with Methylphenidate Hydrochloride tablets
Table 1 presents clinically important drug interactions with methylphenidate hydrochloride tablets.
Table 1: Clinically Important Drug Interactions with Methylphenidate Hydrochloride tablets
Monoamine Oxidase Inhibitors (MAOI)
Clinical Impact
Concomitant use of MAOIs and CNS stimulants, including methylphenidate hydrochloride tablets can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure [see Contraindications (4)].
Intervention
Concomitant use of methylphenidate hydrochloride tablets with MAOIs or within 14 days after discontinuing MAOI treatment is contraindicated.
Antihypertensive Drugs
Clinical Impact
Methylphenidate hydrochloride tablets may decrease the effectiveness of drugs used to treat hypertension [see Warnings and Precautions (5.3)].
Intervention
Monitor blood pressure and adjust the dosage of the antihypertensive drug as needed.
Halogenated Anesthetics
Clinical Impact
Concomitant use of halogenated anesthetics and methylphenidate hydrochloride tablets may increase the risk of sudden blood pressure and heart rate increase during surgery.
Intervention
Avoid use of methylphenidate hydrochloride tablets in patients being treated with anesthetics on the day of surgery.
Risperidone
Clinical Impact
Combined use of methylphenidate with risperidone when there is a change, whether an increase or decrease, in dosage of either or both medications, may increase the risk of extrapyramidal symptoms (EPS)
Intervention
Monitor for signs of EPS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Published studies and postmarketing reports on methylphenidate use during pregnancy have not identified a drug- associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There may be risks to the fetus associated with the use of CNS stimulants use during pregnancy (see Clinical Considerations).
No effects on morphological development were observed in embryo-fetal development studies with oral administration of methylphenidate to pregnant rats and rabbits during organogenesis at doses up to 10 and 15 times, respectively, the maximum recommended human dose (MRHD) of 60 mg/day given to adolescents on a mg/m2 basis. However, spina bifida was observed in rabbits at a dose 52 times the MRHD given to adolescents. A decrease in pup body weight was observed in a pre- and post-natal development study with oral administration of methylphenidate to rats throughout pregnancy and lactation at doses 6 times the MRHD given to adolescents (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
CNS stimulants, such as methylphenidate hydrochloride tablets, can cause vasoconstriction and thereby decrease placental perfusion. No fetal and/or neonatal adverse reactions have been reported with the use of therapeutic doses of methylphenidate during pregnancy; however, premature delivery and low birth weight infants have been reported in amphetamine-dependent mothers.
Data
Animal Data
In embryo-fetal development studies conducted in rats and rabbits, methylphenidate was administered orally at doses of up to 75 and 200 mg/kg/day, respectively, during the period of organogenesis. Malformations (increased incidence of fetal spina bifida) were observed in rabbits at the highest dose, which is approximately 52 times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis. The no effect level for embryo-fetal development in rabbits was 60 mg/kg/day (15times the MRHD given to adolescents on a mg/m2 basis). There was no evidence of morphological development effects in rats, although increased incidences of fetal skeletal variations were seen at the highest dose level (10 times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis), which was also maternally toxic. The no effect level for embryo-fetal development in rats was 25 mg/kg/day (3 times the MRHD on a mg/m2 basis). When methylphenidate was administered to rats throughout pregnancy and lactation at doses of up to 45 mg/kg/day, offspring body weight gain was decreased at the highest dose (6 times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis), but no other effects on postnatal development were observed. The no effect level for pre- and postnatal development in rats was 15 mg/kg/day (~2 times the MRHD given to adolescents on a mg/m2 basis).
8.2 Lactation
Limited published literature, based on milk sampling from seven mothers reports that methylphenidate is present in human milk, which resulted in infant doses of 0.16% to 0.7% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.1 and 2.7. There are no reports of adverse effects on the breastfed infant and no effects on milk production. Long-term neurodevelopmental effects on infants from stimulant exposure are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for methylphenidate hydrochloride tablets and any potential adverse effects on the breastfed infant from methylphenidate hydrochloride tablets or from the underlying maternal condition.
Clinical Considerations
Monitor breastfeeding infants for adverse reactions, such as agitation, insomnia, anorexia, and reduced weight gain.
8.4 Pediatric Use
The safety and effectiveness of methylphenidate hydrochloride tablets for the treatment of ADHD have been established in pediatric patients 6 to 17 years.
The safety and effectiveness of methylphenidate hydrochloride tablets in pediatric patients less than 6 years have not been established. The long-term efficacy of methylphenidate hydrochloride tablets in pediatric patients has not been established.
Long-Term Suppression of Growth
Growth should be monitored during treatment with stimulants, including methylphenidate hydrochloride tablets. Pediatric patients who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.7)].
Juvenile Animal Toxicity Data
Rats treated with methylphenidate early in the postnatal period through sexual maturation demonstrated a decrease in spontaneous locomotor activity in adulthood. A deficit in acquisition of a specific learning task was observed in females only. The doses at which these findings were observed are at least 4 times the MRHD of 60 mg/day given to children on a mg/m2 basis.
In a study conducted in young rats, methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (postnatal Day 7) and continuing through sexual maturity (postnatal Week 10). When these animals were tested as adults (postnatal Weeks 13 to 14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day (approximately 4 times the MRHD of 60 mg/day given to children on a mg/m2 basis) or greater, and a deficit in the acquisition of a specific learning task was seen in females exposed to the highest dose (8 times the MRHD given to children on a mg/m2 basis). The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day (approximately 0.5 times the MRHD given to children on a mg/m2 basis). The clinical significance of the long-term behavioral effects observed in rats is unknown.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Methylphenidate hydrochloride tablets contain methylphenidate hydrochloride, a Schedule II controlled substance.
9.2 Abuse
Methylphenidate hydrochloride tablets has a high potential for abuse and misuse which can lead to the development of a substance use disorder, including addiction [see Warnings and Precautions (5.1)]. Methylphenidate hydrochloride tablets can be diverted for non-medical use into illicit channels or distribution.
Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Misuse and abuse of methylphenidate hydrochloride may cause increased heart rate, respiratory rate, or blood pressure; sweating; dilated pupils; hyperactivity; restlessness; insomnia; decreased appetite; loss of coordination; tremors; flushed skin; vomiting; and/or abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed with CNS stimulants abuse and/or misuse. Misuse and abuse of CNS stimulants, including methylphenidate hydrochloride tablets, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
9.3 Dependence
Methylphenidate hydrochloride tablets may produce physical dependence. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug.
Withdrawal signs and symptoms after abrupt discontinuation or dose reduction following prolonged use of CNS stimulants including methylphenidate hydrochloride tablets include dysphoric mood; depression; fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.
Tolerance
Methylphenidate hydrochloride tablets may produce tolerance. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose).
- Double click here to open Word Application.
- Write unordered list in to word document.
- Close Word Application.
-
10 OVERDOSAGE
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Consider the possibility of multiple drug ingestion. Because methylphenidate has a large volume of distribution and is rapidly metabolized, dialysis is not useful. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
-
11 DESCRIPTION
Methylphenidate hydrochloride tablets contains methylphenidate hydrochloride, a CNS stimulant. It is available as tablets of 5 mg, 10 mg, and 20 mg strength for oral administration.
Methylphenidate hydrochloride is methyl α-phenyl-2-piperidineacetate hydrochloride, and its structural formula is:
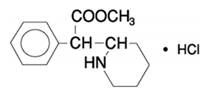
Methylphenidate hydrochloride, USP is a white to off-white fine crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77 g/mol.
Methylphenidate hydrochloride tablets contains the following inactive ingredients: D&C Yellow No. 10 (5-mg and 20-mg tablets), lactose monohydrate, microcrystalline cellulose, sodium starch glycolate (Potato), magnesium stearate, and talc.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Methylphenidate hydrochloride is a CNS stimulant. The mode of therapeutic action in ADHD and narcolepsy is not known.
12.2 Pharmacodynamics
Methylphenidate is a racemic mixture comprised of the d- and l-threo enantiomers. The d-threo enantiomer is more pharmacologically active than the l-threo enantiomer. Methylphenidate blocks the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
Cardiac Electrophysiology
A formal QT study has not been conducted in patients taking methylphenidate hydrochloride tablets.
The effect of dexmethylphenidate, the pharmacologically active d-enantiomer of methylphenidate hydrochloride tablets, on the QT interval was evaluated in a double-blind, placebo- and open-label active (moxifloxacin)-controlled study following single doses of dexmethlyphenidate XR 40 mg (maximum recommended adult total daily dosage) in 75 healthy volunteers.
Electrocardiograms were collected up to 12 hours postdose. Frederica's method for heart rate correction was employed to derive the corrected QT interval (QTcF). The maximum mean prolongation of QTcF intervals was less than 5 ms, and the upper limit of the 90% confidence interval was below 10 ms for all time-matched comparisons versus placebo. This was below the threshold of clinical concern and there was no evident exposure response relationship.
12.3 Pharmacokinetics
The time to peak rate in children was 1.9 hours (0.3 to 4.4 hours) for the methylphenidate hydrochloride tablets.
Effect of Food
After a high-fat meal, both area under the curve (AUC) (by 25 %) and Cmax (by 27 %) are higher. Time to Cmax (Tmax) is faster after a high-fat meal (median Tmax: 2.5 hours) as compared to without food (median Tmax: 3 hours).
Distribution
Binding to plasma proteins is low (10% to 33%). The volume of distribution was 2.65 ± 1.11 L/kg for d- methylphenidate and 1.80 ± 0.91 L/kg for l- methylphenidate.
Elimination
The systemic clearance is 0.40 ± 0.12 L/h/kg for d-methylphenidate and 0.73 ± 0.28 L/h/kg for l-methylphenidate.
Metabolism
Methylphenidate is metabolized primarily by de-esterification to alpha-phenyl-piperidine acetic acid (ritalinic acid), which has little or no pharmacologic activity.
Excretion
After oral administration, 78% to 97% of the dose is excreted in the urine and 1% to 3% in feces in the form of metabolites within 48 to 96 hours. Most of the dose is excreted in the urine as alpha-phenyl-2-piperidine acetic acid (60% to 86%). The cumulative urinary excretion of alpha-phenyl-2-piperidine acetic acid are not significantly different for methylphenidate hydrochloride extended-release tablets.
Studies in Specific Populations
Male and Female Patients
In a clinical study involving adult subjects who received methylphenidate hydrochloride extended-release tablets, plasma concentrations of methylphenidate hydrochloride's major metabolite appeared to be greater in females than in males. No gender differences were observed for methylphenidate hydrochloride plasma concentration in the same subjects.
Racial or Ethnic Groups
There is insufficient experience with the use of methylphenidate hydrochloride tablets to detect ethnic variations in pharmacokinetics.
Patients with Renal Impairment
Methylphenidate hydrochloride tablets have not been studied in renally-impaired patients. Renal impairment is expected to have minimal effect on the pharmacokinetics of methylphenidate since less than 1% of a radiolabeled dose is excreted in the urine as unchanged compound, and the major metabolite (ritalinic acid), has little or no pharmacologic activity.
Patients with Hepatic Impairment
Methylphenidate hydrochloride tablets have not been studied in patients with hepatic impairment. Hepatic impairment is expected to have minimal effect on the pharmacokinetics of methylphenidate since it is metabolized primarily to ritalinic acid by nonmicrosomal hydrolytic esterases that are widely distributed throughout the body.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
In a lifetime carcinogenicity study carried out in B6C3F1 mice, methylphenidate caused an increase in hepatocellular adenomas, and in males only, an increase in hepatoblastomas at a daily dose of approximately 60 mg/kg/day. This dose is approximately 2 times the MRHD of 60 mg/day given to children on mg/m2 basis. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors and the significance of these results to humans is unknown.
Methylphenidate did not cause any increase in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 4 times the MRHD (children) on a mg/m2 basis.
In a 24-week carcinogenicity study in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Male and female mice were fed diets containing the same concentration of methylphenidate as in the lifetime carcinogenicity study; the high-dose groups were exposed to 60 to 74 mg/kg/day of methylphenidate.
Mutagenesis
Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay, in the in vitro mouse lymphoma cell forward mutation assay, or in the in vitro chromosomal aberration assay using human lymphocytes. Sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an in vitro assay in cultured Chinese Hamster Ovary cells. Methylphenidate was negative in vivo in males and females in the mouse bone marrow micronucleus assay.
Impairment of Fertility
No human data on the effect of methylphenidate on fertility are available. Methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week continuous breeding study. The study was conducted at doses up to 160 mg/kg/day, approximately 10times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Methylphenidate Hydrochloride Tablets, USP are available as follows
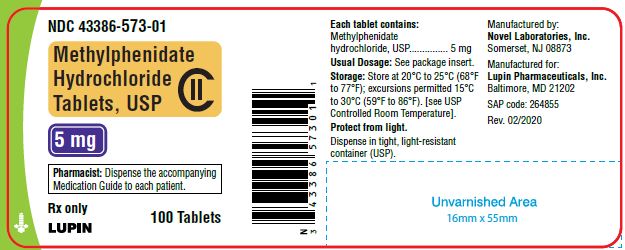
5 mg Tablets - light yellow colored, round, flat face tablets with "n above 573" debossed on one side and plain on the other side, supplied as:
Bottles of 30 ……………………………........NDC 43386-573-03
Bottles of 100………………………………...NDC 43386-573-01
Bottles of 500 ……………………………......NDC 43386-573-05
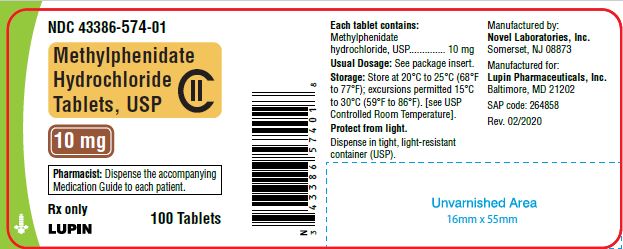
10 mg Tablets - white to off white colored, round, flat face tablets with "n above 574" debossed on one side and bisected line debossed on the other side, supplied as:
Bottles of 30 ……………………………...... NDC 43386-574-03
Bottles of 100………………………………...NDC 43386-574-01
Bottles of 500 ……………………………......NDC 43386-574-05
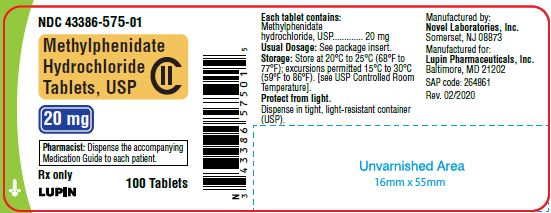
20 mg Tablets - light yellow colored, round biconvex tablets with "n above 575" debossed on one side and bisect line debossed on the other side, supplied as:
Bottles of 30 ……………………………........NDC 43386-575-03
Bottles of 100………………………………...NDC 43386-575-01
Bottles of 500 ……………………………......NDC 43386-575-05
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15ºC and 30ºC (59ºF and 86ºF) [see USP Controlled Room Temperature].
Protect from light.
Dispense in tight, light-resistant container (USP).
Disposal
Comply with local laws and regulations on drug disposal of CNS stimulants. Dispose of remaining, unused, or expired methylphenidate hydrochloride tablets by a medicine takeback program or by an authorized collector registered with the Drug Enforcement Administration. If no take-back program or authorized collector is available, mix methylphenidate hydrochloride tablets with an undesirable, nontoxic substance to make it less appealing to children and pets. Place the mixture in a container, such as a sealed plastic bag and discard methylphenidate hydrochloride tablets in the household trash.
-
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Controlled Substance Status/High Potential for Abuse and Dependence
Advise patients that methylphenidate hydrochloride tablets are controlled substances, and they can be abused and lead to dependence. Instruct patients that they should not give methylphenidate hydrochloride tablets to anyone else. Advise patients to store methylphenidate hydrochloride tablets in a safe place, preferably locked, to prevent abuse. Advise patients to comply with laws and regulations on drug disposal. Advise patients to dispose of remaining, unused, or expired methylphenidate hydrochloride tablets by a medicine take-back program if available [see Boxed Warning, Warnings and Precautions (5.1), Drug Abuse and Dependence (9.1, 9.2, 9.3), How Supplied/Storage and Handling (16)].
Serious Cardiovascular Risks
Advise patients that there is a potential serious cardiovascular risk, including sudden death, myocardial infarction, stroke, and hypertension with methylphenidate hydrochloride tablets use. Instruct patients to contact a healthcare provider immediately if they develop symptoms, such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease [see Warnings and Precautions (5.2)].
Blood Pressure and Heart Rate Increases
Instruct patients that methylphenidate hydrochloride tablets can cause elevations of their blood pressure and pulse rate [see Warnings and Precautions (5.3)].
Psychiatric Risks
Advise patients that methylphenidate hydrochloride tablets, at recommended doses, can cause psychotic or manic symptoms, even in patients without prior history of psychotic symptoms or mania [see Warnings and Precautions (5.4)].
Priapism
Advise patients of the possibility of painful or prolonged penile erections (priapism). Instruct them to seek immediate medical attention in the event of priapism [see Warnings and Precautions (5.5)].
Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, Including Raynaud's Phenomenon]
Instruct patients about the risk of peripheral vasculopathy, including Raynaud's phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red. Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking methylphenidate hydrochloride tablets. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients [see Warnings and Precautions (5.6)].
Suppression of Growth
Advise patients that methylphenidate hydrochloride tablets may cause slowing of growth and weight loss [see Warnings and Precautions (5.7)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in patients exposed to ADHD medications, including methylphenidate hydrochloride tablets, during pregnancy [see Use in Specific Populations (8.1)].
Manufactured by:
Novel Laboratories, Inc.
Somerset, NJ 08873
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, MD 21202
SAP Code: 275056
Rev. 11/2023
-
SPL MEDGUIDE
Methylphenidate Hydrochloride
(METH-il-FEN-i-date HYE-droe-KLOR-ide)
Tablets, USP CII
Rx Only
What is the most important information I should know about methylphenidate hydrochloride tablets?
Methylphenidate hydrochloride tablets may cause serious side effects, including:
- Abuse, misuse, and addiction. Methylphenidate hydrochloride tablets has a high chance for abuse and misuse and may lead to substance use problems, including addiction. Misuse and abuse of methylphenidate hydrochloride tablets, other methylphenidate containing medicines, and amphetamine containing medicines, can lead to overdose and death. The risk of overdose and death is increased with higher doses of methylphenidate hydrochloride tablets or when it is used in ways that are not approved, such as snorting or injection.
- Your healthcare provider should check you or your child's risk for abuse, misuse, and addiction before starting treatment with methylphenidate hydrochloride tablets and will monitor you or your child during treatment.
- Methylphenidate hydrochloride tablets may lead to physical dependence after prolonged use, even if taken as directed by your healthcare provider.
- Do not give methylphenidate hydrochloride tablets to anyone else. See "What is methylphenidate hydrochloride tablets?"for more information.
- Keep methylphenidate hydrochloride tablets in a safe place and properly dispose of any unused medicine. See "How should I store methylphenidate hydrochloride tablets?"for more information.
- Tell your healthcare provider if you or your child have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
- Risks for people with serious heart disease. Sudden death has happened in people who have heart defects or other serious heart disease.
Your healthcare provider should check you or your child carefully for heart problems before starting methylphenidate hydrochloride tablets.
Tell your healthcare provider if you or your child have any heart problems, heart disease, or heart defects.
Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child has any signs of heart problems, such as chest pain, shortness of breath, or fainting while taking methylphenidate hydrochloride tablets.
- Increased blood pressure and heart rate.
- Your healthcare provider should check you or your child's blood pressure and heart rate regularly during treatment with methylphenidate hydrochloride tablets.
- Mental (psychiatric) problems:
- All Patients
- new or worse behavior and thought problems
- new or worse bipolar illness
- new psychotic symptoms (such as hearing voices, believing things that are not true, are suspicious) or new manic symptoms
Tell your healthcare provider about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression.
Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems while taking methylphenidate hydrochloride tablets, especially seeing or hearing things that are not real, believing things that are not real, or are suspicious.
What is methylphenidate hydrochloride tablets?
- Methylphenidate hydrochloride tablets are a central nervous system (CNS) stimulant prescription medicine. It is used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). Methylphenidate hydrochloride tablets may help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD.
- Methylphenidate hydrochloride tablets should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
- Methylphenidate hydrochloride tablets are also used in the treatment of a sleep disorder called narcolepsy.
Methylphenidate hydrochloride tablets is a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep methylphenidate hydrochloride tablets in a safe place to protect it from theft.
Never give your methylphenidate hydrochloride tablets to anyone else, because it may cause death or harm them. Selling or giving away methylphenidate hydrochloride tablets may harm others and is against the law.
Methylphenidate hydrochloride tablets should not be taken if you or your child:
- are allergic to methylphenidate hydrochloride, or any of the ingredients in methylphenidate hydrochloride tablets. See the end of this Medication Guide for a complete list of ingredients in methylphenidate hydrochloride tablets.
- are taking or have taken within the past 14 days an anti-depression medicine called a monoamine oxidase inhibitor or (MAOI).
Methylphenidate hydrochloride tablets may not be right for you or your child. Before starting methylphenidate hydrochloride tablets, tell your or your child's doctor about all health conditions (or a family history of) including:
- heart problems, heart disease, heart defects, high blood pressure
- mental problems, including psychosis, mania, bipolar illness, or depression
- circulation problems in fingers or toes
- have eye problems, including increased pressure in your eye, glaucoma, or problems with your close-up vision (farsightedness)
- have or had repeated movements or sounds (tics) or Tourette's syndrome, or have a family history of tics or Tourette's syndrome.
- if you are pregnant or plan to become pregnant. It is not known if methylphenidate hydrochloride tablets will harm your unborn baby.
- if you are breastfeeding or plan to breastfeed. methylphenidate hydrochloride passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with methylphenidate hydrochloride tablets.
Tell your healthcare provider about all of the medicines that you or your child takes including prescription and over-the- counter medicines, vitamins, and herbal supplements. Methylphenidate hydrochloride tablets and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking methylphenidate hydrochloride tablets.
Your healthcare provider will decide whether methylphenidate hydrochloride tablets can be taken with other medicines.
Especially tell your healthcare provider if you or your child takes:
- anti-depression medicines including MAOIs
- blood pressure medicines (anti-hypertensive)
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your healthcare provider and pharmacist.
- You should not take methylphenidate hydrochloride tablets on the day of your operation if a certain type of anesthetic is used. This is because there is a chance of a sudden rise in blood pressure and heart rate during the operation.
Do not start any new medicine while taking methylphenidate hydrochloride tablets without talking to your healthcare provider first.
How should methylphenidate hydrochloride tablets be taken?
- Take methylphenidate hydrochloride tablets exactly as prescribed. Your healthcare provider may adjust the dose until it is right for you or your child.
- Methylphenidate hydrochloride tablets are usually taken 2 to 3 times a day.
- Take methylphenidate hydrochloride tablets 30 to 45 minutes before a meal.
- Your healthcare provider may do regular checks of the blood, heart, and blood pressure while taking methylphenidate hydrochloride tablets. Children should have their height and weight checked often while taking methylphenidate hydrochloride tablets. If you or your child take too much methylphenidate hydrochloride tablets, call your healthcare provider or Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What are the possible side effects of methylphenidate hydrochloride tablets?
Methylphenidate hydrochloride tablets may cause serious side effects, including:
- see "What is the most important information I should know about methylphenidate hydrochloride tablets?" for information on reported heart and mental problems.
- painful and prolonged erections (priapism) have occurred with methylphenidate. If you or your child develops priapism, seek medical help right away. Because of the potential for lasting damage, priapism should be evaluated by a healthcare provider immediately.
- circulation problems in fingers and toes (peripheral vasculopathy, including Raynaud's phenomenon):
- fingers or toes may feel numb, cool, painful
- fingers or toes may change color from pale, to blue, to red
- Tell your healthcare provider if you or your child have, numbness, pain, skin color change, or sensitivity to temperature in the fingers or toes.
- Call your healthcare provider right away if you have or your child has any signs of unexplained wounds appearing on fingers or toes while taking methylphenidate hydrochloride tablets.
- slowing of growth (height and weight) in children
- Common side effects include:
- fast heart beat
- headache
- nervousness
- decreased appetite
- nausea
- abnormal heartbeat
- (palpitations)
- trouble sleeping
- sweating a lot
- dry mouth
- stomach pain
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store methylphenidate hydrochloride tablets?
- Store methylphenidate hydrochloride tablets in a safe place and in a tightly closed container at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect from light.
- Dispose of remaining, unused, or expired methylphenidate hydrochloride tablets by a medicine take-back program at a U.S. Drug Enforcement Administration (DEA) authorized collection site. If no take-back program or DEA authorized collector is available, mix methylphenidate hydrochloride tablets with an undesirable, nontoxic substance such as dirt, cat litter, or used coffee grounds to make it less appealing to children and pets. Place the mixture in a container, such as a sealed plastic bag and throw away (discard) methylphenidate hydrochloride tablets in the household trash. Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
- Keep methylphenidate hydrochloride tablets and all medicines out of the reach of children.
General information about the safe and effective use of methylphenidate hydrochloride tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about methylphenidate hydrochloride tablets that is written for healthcare professionals. Do not use methylphenidate hydrochloride tablets for a condition for which it was not prescribed. Do not give methylphenidate hydrochloride tablets to other people, even if they have the same symptoms that you have. It may harm them and it is against the law.
What are the ingredients in methylphenidate hydrochloride tablets?
Active ingredient: methylphenidate HCl
Inactive ingredients: D&C Yellow No. 10 (5-mg and 20-mg tablets), lactose monohydrate, microcrystalline cellulose, sodium starch glycolate (potato), magnesium stearate, and talc
Manufactured by:
Novel Laboratories, Inc.
Somerset, NJ 08873
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, MD 21202
SAP Code: 275055
Rev. 11/2023
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 11/2023
For more information, call 1-866-403-7592
-
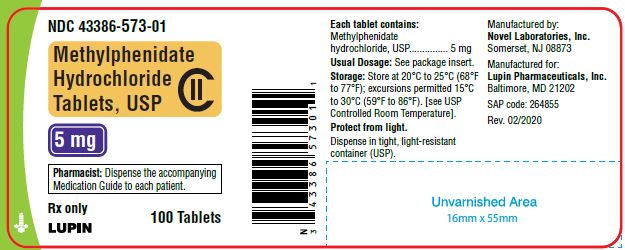
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Methylphenidate Hydrochloride Tablets, USP 5 mg
100 Tablets
NDC 43386-573-01
5 mg-100 ct container
5 mg-100 ct container
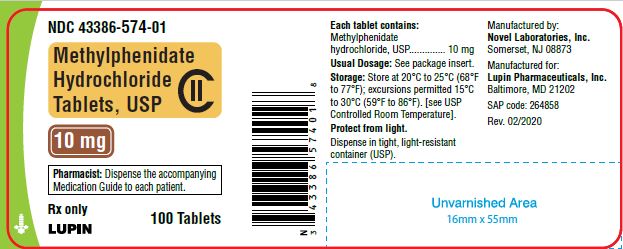
Methylphenidate Hydrochloride Tablets, USP 10 mg
100 Tablets
NDC 43386-574-01
10 mg-100 ct container
10 mg-100 ct container
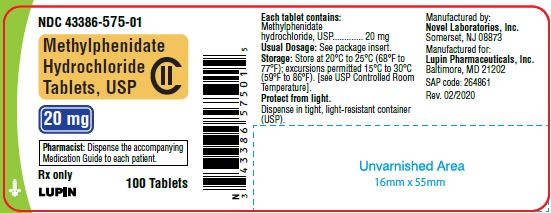
Methylphenidate Hydrochloride Tablets, USP 20 mg
100 Tablets
NDC 43386-575-01
20 mg-100 ct container
20 mg-100 ct container
-
INGREDIENTS AND APPEARANCE
METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43386-573 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLPHENIDATE HYDROCHLORIDE (UNII: 4B3SC438HI) (METHYLPHENIDATE - UNII:207ZZ9QZ49) METHYLPHENIDATE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color YELLOW Score no score Shape ROUND Size 6mm Flavor Imprint Code nabove573 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43386-573-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/20/2016 2 NDC:43386-573-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2040 3 NDC:43386-573-03 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2040 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207884 01/20/2016 METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43386-574 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLPHENIDATE HYDROCHLORIDE (UNII: 4B3SC438HI) (METHYLPHENIDATE - UNII:207ZZ9QZ49) METHYLPHENIDATE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 7mm Flavor Imprint Code nabove574 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43386-574-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/20/2016 2 NDC:43386-574-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2040 3 NDC:43386-574-03 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2040 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207884 11/13/2015 METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43386-575 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLPHENIDATE HYDROCHLORIDE (UNII: 4B3SC438HI) (METHYLPHENIDATE - UNII:207ZZ9QZ49) METHYLPHENIDATE HYDROCHLORIDE 20 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color YELLOW Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code nabove575 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43386-575-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/20/2016 2 NDC:43386-575-03 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2040 3 NDC:43386-575-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2040 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207884 11/13/2015 Labeler - Lupin Pharmaceuticals,Inc. (089153071) Registrant - Novel Laboratories, Inc. (793518643) Establishment Name Address ID/FEI Business Operations Novel Laboratories, Inc. 793518643 ANALYSIS(43386-573, 43386-574, 43386-575) , MANUFACTURE(43386-573, 43386-574, 43386-575) , PACK(43386-573, 43386-574, 43386-575)