Label: CIMETIDINE tablet, film coated
- NDC Code(s): 59651-823-01, 59651-824-01, 59651-825-01, 59651-825-05, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
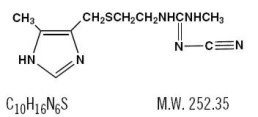
DESCRIPTIONCimetidine is a histamine H2-receptor antagonist. Chemically it is N"-cyano-N-methyl-N'-[2-[[(5-methyl-1H-imidazol-4-yl)methyl]thio]-ethyl]guanidine. Its structural formula is: Cimetidine ...
-
CLINICAL PHARMACOLOGYCimetidine tablets competitively inhibits the action of histamine at the histamine H2 receptors of the parietal cells and thus is a histamine H2-receptor antagonist. Cimetidine is not an ...
-
CLINICAL TRIALSDuodenal Ulcer - Cimetidine tablets have been shown to be effective in the treatment of active duodenal ulcer and, at reduced dosage, in maintenance therapy following healing of active ...
-
INDICATIONS AND USAGECimetidine tablets are indicated in: 1. Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks and there is rarely reason to use cimetidine tablets at full ...
-
CONTRAINDICATIONSCimetidine tablets are contraindicated for patients known to have hypersensitivity to the product.
-
PRECAUTIONSGeneral - Rare instances of cardiac arrhythmias and hypotension have been reported following the rapid administration of cimetidine hydrochloride injection by intravenous bolus. Symptomatic ...
-
ADVERSE REACTIONSAdverse effects reported in patients taking cimetidine tablets are described as follows by body system. Incidence figures of 1 in 100 and greater are generally derived from controlled clinical ...
-
OVERDOSAGEStudies in animals indicate that toxic doses are associated with respiratory failure and tachycardia that may be controlled by assisted respiration and the administration of a ...
-
DOSAGE AND ADMINISTRATIONDuodenal Ulcer - Active Duodenal Ulcer - Clinical studies have indicated that suppression of nocturnal acid is the most important factor in duodenal ulcer healing (see CLINICAL PHARMACOLOGY ...
-
HOW SUPPLIEDCimetidine Tablets, USP are available containing 200 mg, 300 mg, 400 mg or 800 mg of cimetidine, USP. The 200 mg tablets are green colored, film-coated oval shaped tablets, debossed with C 2 on ...
-
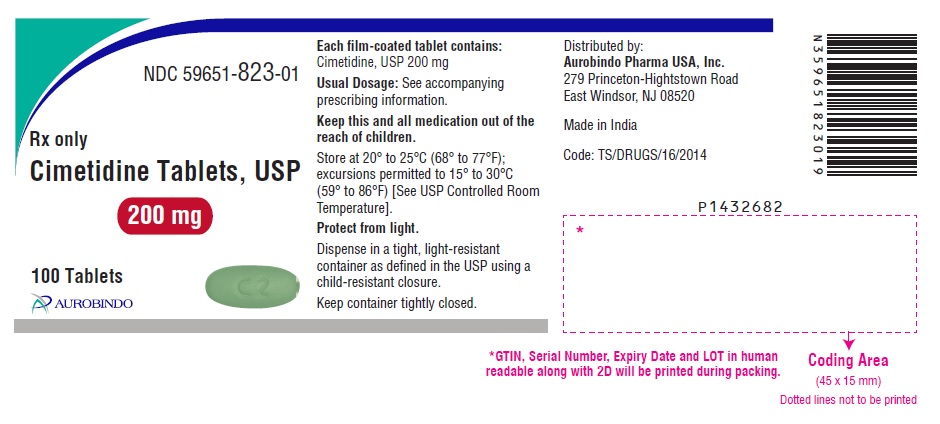
PACKAGE LABEL PRINCIPAL DISPLAY PANEL - 200 mg (100 Tablets Bottle)NDC 59651-823-01 - Rx only - Cimetidine Tablets, USP - 200 mg - 100 Tablets - AUROBINDO
-
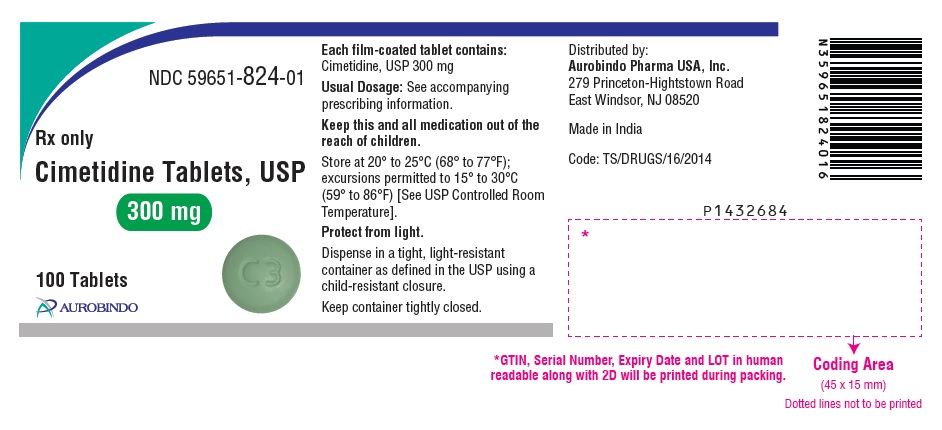
PACKAGE LABEL PRINCIPAL DISPLAY PANEL - 300 mg (100 Tablets Bottle)NDC 59651-824-01 - Rx only - Cimetidine Tablets, USP - 300 mg - 100 Tablets - AUROBINDO
-
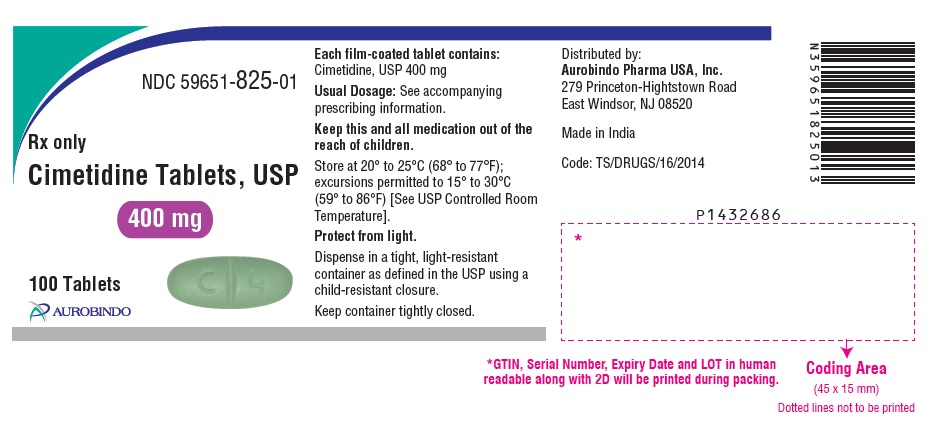
PACKAGE LABEL PRINCIPAL DISPLAY PANEL - 400 mg (100 Tablets Bottle)NDC 59651-825-01 - Rx only - Cimetidine Tablets, USP - 400 mg - 100 Tablets - AUROBINDO
-
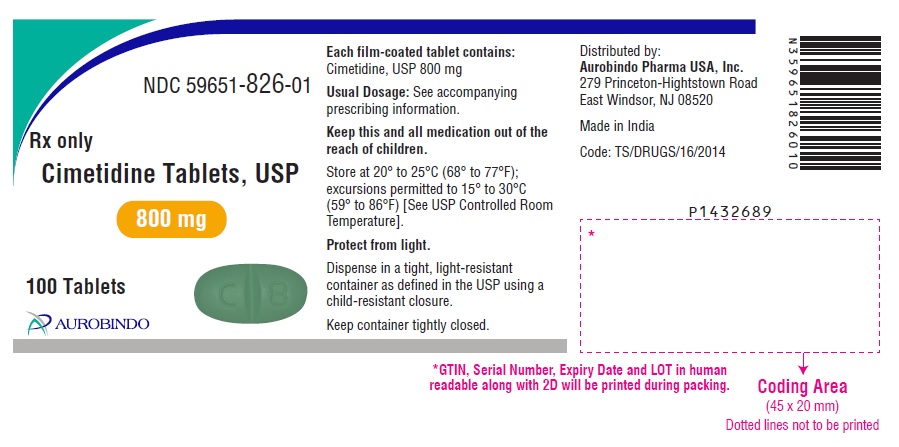
PACKAGE LABEL PRINCIPAL DISPLAY PANEL - 800 mg (100 Tablets Bottle)NDC 59651-826-01 - Rx only - Cimetidine Tablets, USP - 800 mg - 100 Tablets - AUROBINDO
-
INGREDIENTS AND APPEARANCEProduct Information