Label: AMLODIPINE BESYLATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 10135-759-10, 10135-759-90, 10135-760-10, 10135-760-90, view more - Packager: Marlex Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMLODIPINE BESYLATE TABLETS safely and effectively. See full prescribing information for AMLODIPINE BESYLATE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Hypertension - Amlodipine besylate tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal ...
-
2 DOSAGE AND ADMINISTRATION2.1 Adults - The usual initial antihypertensive oral dose of amlodipine besylate tablets is 5 mg once daily, and the maximum dose is 10 mg once daily. Small, fragile, or elderly ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 2.5 mg white to off-white, round, flat-faced, beveled edged tablet engraved with “P41” on one side and plain on the other side. Tablets: 5 mg white to off-white, round, flat-faced ...
-
4 CONTRAINDICATIONSAmlodipine besylate tablets are contraindicated in patients with known sensitivity to amlodipine.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. Because of the gradual onset of action, acute hypotension is unlikely. 5.2 ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.2 Impact of Amlodipine on Other Drugs - Simvastatin - Co-administration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data based on post-marketing reports with amlodipine besylate use in pregnant women are not sufficient to inform a drug-associated risk for ...
-
10 OVERDOSAGEOverdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. In humans, experience with intentional overdosage of amlodipine ...
-
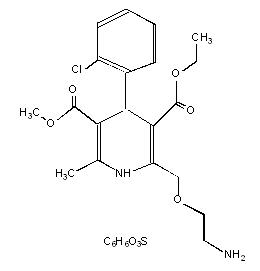
11 DESCRIPTIONAmlodipine besylate tablets, USP is the besylate salt of amlodipine, a long-acting calcium channel blocker. Amlodipine besylate, USP is chemically described as 3-Ethyl-5-methyl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into vascular ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Rats and mice treated with amlodipine maleate in the diet for up to two years, at concentrations calculated to provide daily dosage ...
-
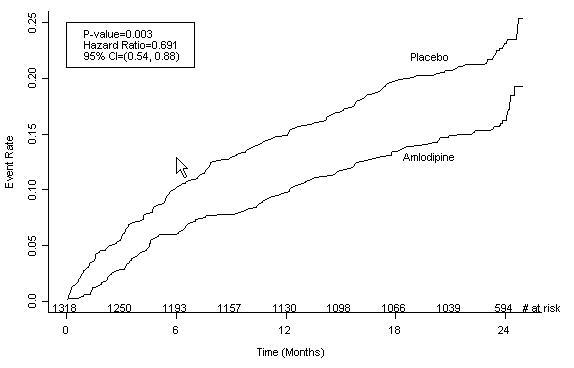
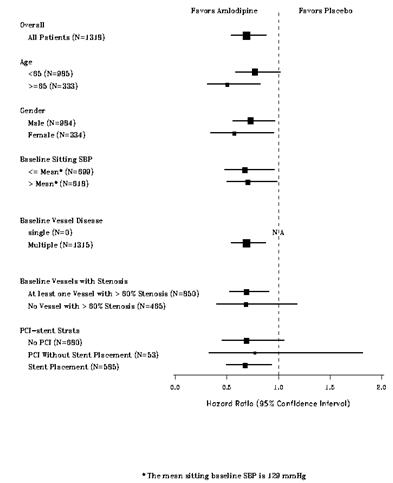
14 CLINICAL STUDIES14.1 Effects in Hypertension - Adult Patients - The antihypertensive efficacy of amlodipine besylate tablets has been demonstrated in a total of 15 double-blind ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING2.5 mg Tablets - Amlodipine Besylate Tablets, USP 2.5 mg (amlodipine besylate, USP equivalent to 2.5 mg of amlodipine per tablet) are supplied as white to off-white, round, flat-faced, beveled ...
-
PATIENT PACKAGE INSERTPatient Information - Amlodipine Besylate Tablets, USP - Read this information carefully before you start taking - amlodipine besylate tablets and each time you refill your prescription. There may ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANELNDC 10135-759-90 - Amlodipine Besylate Tablets, USP - 2.5 mg* Rx only - 90 Tablets - NDC 10135-759-10 - Amlodipine Besylate Tablets, USP - 2.5 mg* Rx only - 1000 ...
-
INGREDIENTS AND APPEARANCEProduct Information