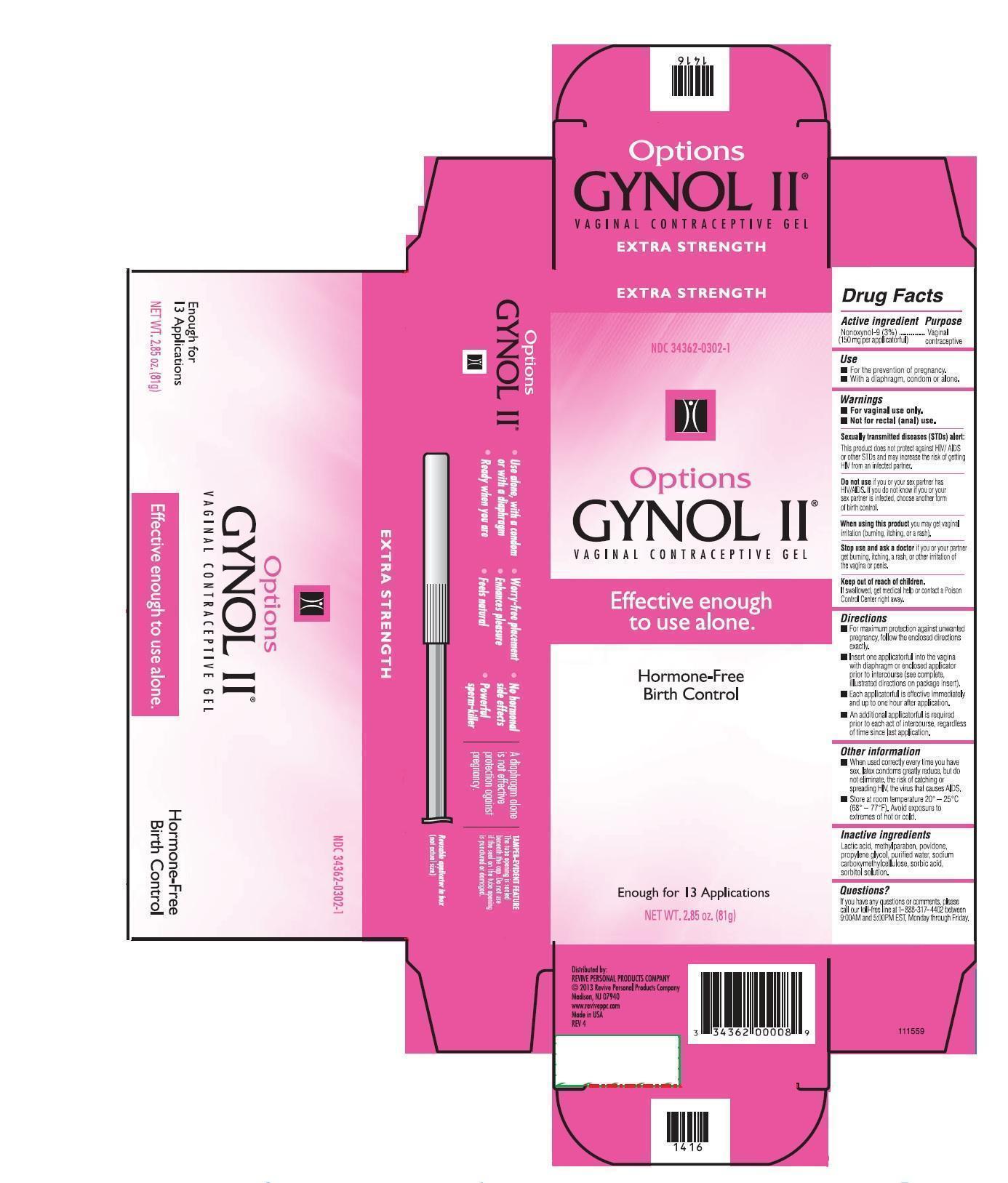
Label: GYNOL II EXTRA STRENGTH- nonoxynol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 34362-0302-1, 34362-0302-5 - Packager: Caldwell Consumer Health LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredientNonoxynol-9 (3%) (100 mg per applicatorful)............................................Vaginal Contraceptive
-
UsesFor the prevention of pregnancy. With condom or alone.
-
WARNINGSFor vaginal use only. Not for rectal (anal) use. Sexually transmitted diseases (STDs) alert: This product does not protect against HIV/AIDS or other STDs and may increase the risk of getting HIV ...
-
DirectionsFor maximum protection against unwanted pregnancy, follow the enclosed directions exactly. Each applicatorful is effective immediately and up to one hour after application.
-
DOSAGE & ADMINISTRATIONInsert one applicatorful into the vagina prior to intercourse (see complete, illustrated directions on the package insert). An additional applicatorful is required prior to each act of intercourse ...
-
Other InformationWhen used correctly every time you have sex, latex condoms greatly reduce, but do not eliminate, the risk of catching or spreading HIV, the virus that causes AIDS. Store at room temperature, 20° ...
-
Inactive ingredientsLactic acid, methylparaben, povidone, propylene glycol, purified water, sodium carboxymethylcellulose, sorbic acid, sorbitol solution.
-
PRINCIPAL DISPLAY PANELmm1.jpg - MM2.jpg
-
INGREDIENTS AND APPEARANCEProduct Information