Label: SODIUM BICARBONATE injection, solution
- NDC Code(s): 0409-5555-02, 0409-5555-12
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONInjection, USP - FOR THE CORRECTION OF - METABOLIC ACIDOSIS AND - OTHER CONDITIONS REQUIRING - SYSTEMIC ALKALINIZATION. Glass Vial - Rx ...
-
DESCRIPTIONSodium Bicarbonate Injection, USP is a sterile, nonpyrogenic, hypertonic solution of sodium bicarbonate (NaHCO3) in water for injection for administration by the intravenous route as an ...
-
CLINICAL PHARMACOLOGYIntravenous sodium bicarbonate therapy increases plasma bicarbonate, buffers excess hydrogen ion concentration, raises blood pH and reverses the clinical manifestations of acidosis. Sodium ...
-
INDICATIONS AND USAGESodium Bicarbonate Injection, USP is indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or ...
-
CONTRAINDICATIONSSodium Bicarbonate Injection, USP is contraindicated in patients who are losing chloride by vomiting or from continuous gastrointestinal suction, and in patients receiving diuretics known to ...
-
WARNINGSSolutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema ...
-
PRECAUTIONSGeneral - Do not use unless solution is clear and the container or seal is intact. Discard unused portion. The potentially large loads of sodium given with bicarbonate require that caution be ...
-
ADVERSE REACTIONSOverly aggressive therapy with Sodium Bicarbonate Injection, USP can result in metabolic alkalosis (associated with muscular twitchings, irritability, and tetany) and hypernatremia. Inadvertent ...
-
OVERDOSAGEShould alkalosis result, the bicarbonate should be stopped and the patient managed according to the degree of alkalosis present. 0.9% sodium chloride injection intravenous may be given; potassium ...
-
DOSAGE AND ADMINISTRATIONSodium Bicarbonate Injection, USP is administered by the intravenous route. In cardiac arrest, a rapid intravenous dose of 44.6 to 100 mEq may be given initially and continued at a rate of 44.6 ...
-
HOW SUPPLIEDSodium Bicarbonate Injection, USP is supplied in the following dosage forms. NDC Number (Unit of Sale) 0409-5555-02 - NDC Number (Each) 0409-5555-12 - Dosage Form - Glass ...
-
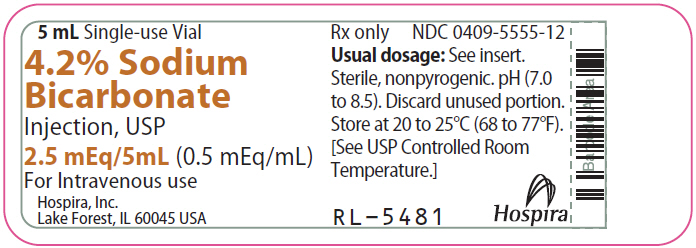
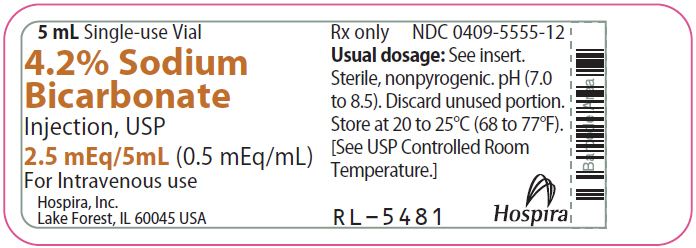
PRINCIPAL DISPLAY PANEL - 5 mL Vial Label5 mL Single-use Vial - 4.2% Sodium - Bicarbonate - Injection, USP - 2.5 mEq/5mL (0.5 mEq/mL) For Intravenous use - Hospira, Inc. Lake Forest, IL 60045 USA - Hospira
-
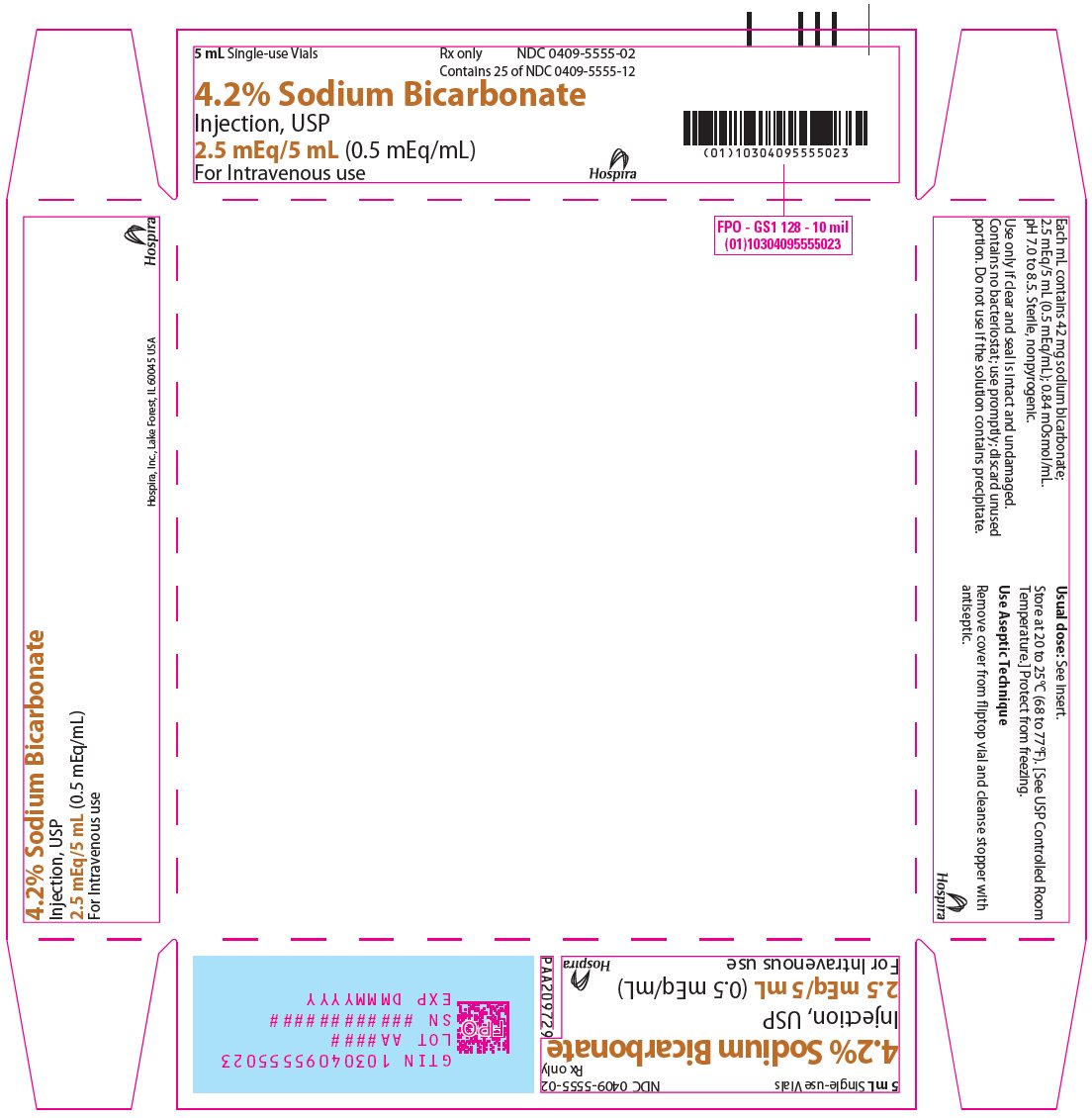
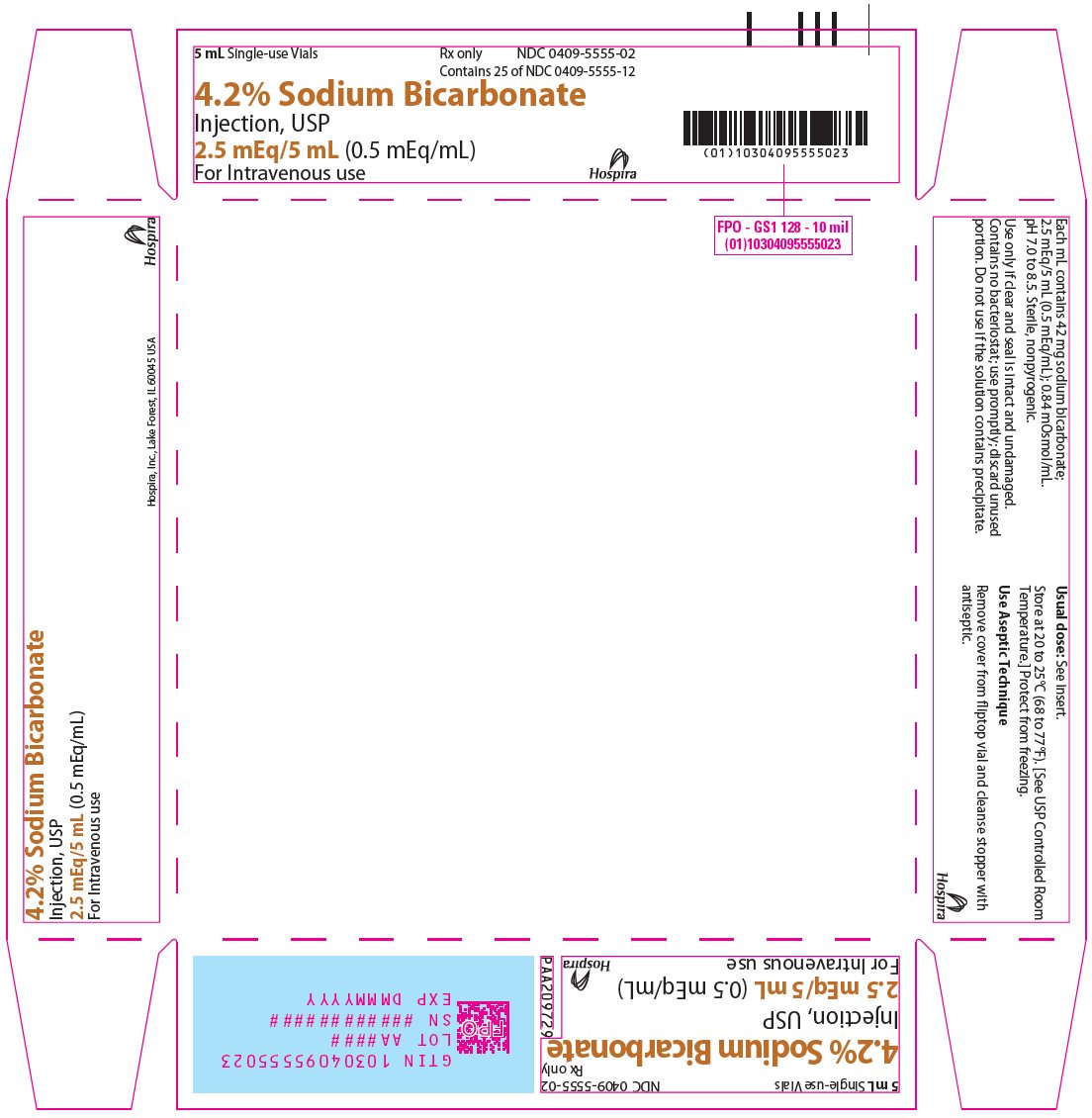
PRINCIPAL DISPLAY PANEL - 5 mL Vial Tray5 mL Single-use Vials - Rx only - NDC 0409-5555-02 - Contains 25 of NDC 0409-5555-12 - 4.2% Sodium Bicarbonate - Injection, USP - 2.5 mEq/5 mL (0.5 mEq/mL) For Intravenous use - Hospira
-
INGREDIENTS AND APPEARANCEProduct Information