Label: XIPERE- triamcinolone acetonide injection, suspension
- NDC Code(s): 24208-040-01, 24208-040-02, 24208-040-40, 24208-040-41
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use XIPERE - ®safely and effectively. See full prescribing information for XIPERE - ®. XIPERE - ®(triamcinolone acetonide injectable ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEXIPERE - ®(triamcinolone acetonide injectable suspension) 40 mg/mL is indicated for the treatment of macular edema associated with uveitis.
-
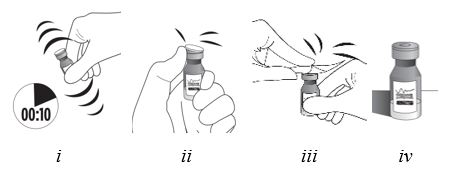
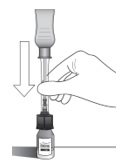
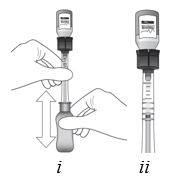
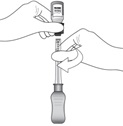
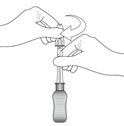
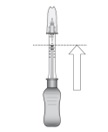
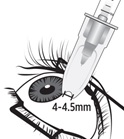
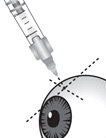
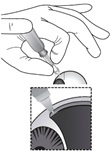
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - For suprachoroidal injection using the SCS Microinjector - ®. The recommended dose of XIPERE - ®is 4 mg (0.1 mL of the 40 mg/mL injectable suspension). 2.2 ...
-
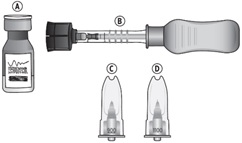
3 DOSAGE FORMS AND STRENGTHSInjectable suspension: triamcinolone acetonide 40 mg/mL suspension in a single-dose glass vial for use with the supplied SCS Microinjector - ®.
-
4 CONTRAINDICATIONS4.1 Ocular or Periocular Infections - XIPERE - ®is contraindicated in patients with active or suspected ocular or periocular infections including most viral diseases of the cornea and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Potential Corticosteroid-Related Effects - Use of corticosteroids may produce cataracts, increased intraocular pressure, and glaucoma. Use of corticosteroids may enhance the establishment of ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies with XIPERE - ®in pregnant women to inform drug-associated risks. In animal reproductive studies from the ...
-
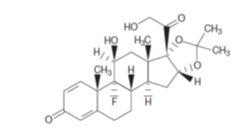
11 DESCRIPTIONXIPERE - ®is a sterile, injectable suspension of triamcinolone acetonide, a synthetic corticosteroid for suprachoroidal use with the SCS Microinjector - ®. Each mL of the aqueous suspension ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Triamcinolone acetonide is a synthetic glucocorticoid (glucocorticoids are often referred to as corticosteroids) with immunosuppressive and anti-inflammatory activity ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No information is available on the carcinogenic potential of triamcinolone acetonide. Mutagenesis - No information is ...
-
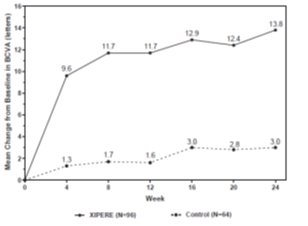
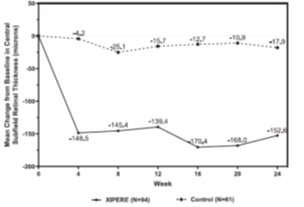
14 CLINICAL STUDIESThe efficacy of XIPERE - ®was assessed in a 6-month, randomized, multicenter, double-masked, sham-controlled study in patients with macular edema associated with anterior-, intermediate- ...
-
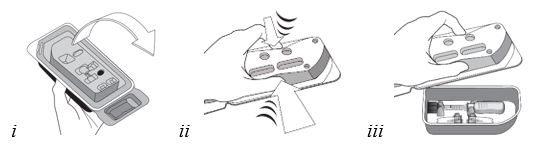
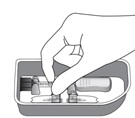
16 HOW SUPPLIED/STORAGE AND HANDLINGXIPERE - ®is supplied with the following sterile components for administration, sealed in a Tyvek covered tray, and one single-dose glass vial, in a carton with a package insert (NDC ...
-
17 PATIENT COUNSELING INFORMATIONCorticosteroid-Related Effects - Advise patients that they may develop elevated intraocular pressure following treatment, which may need to be managed with medication or surgery. When to Seek ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC24208-040-40 - XIPERE - ® (triamcinolone acetonide - injectable suspension) 40 mg/mL - For suprachoroidal use - Carton contains: • One single-dose glass vial of triamcinolone ...
-
INGREDIENTS AND APPEARANCEProduct Information