Label: METAXALONE tablet
- NDC Code(s): 70518-0771-0, 70518-0771-1, 70518-0771-2, 70518-0771-3, view more
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 0527-1435
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METAXALONE safely and effectively. See full prescribing information for METAXALONE. METAXALONE tablets, USP, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMetaxalone is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults and pediatric ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dosage of metaxalone in adults and pediatric patients 13 years of age and older is 800 mg orally three to four times a day - [see Use in Specific Populations ( 8) ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 800 mg pink, capsule shaped, scored tablets, debossed “LCI" on one side and "14" bisect "35" on the other side.
-
4 CONTRAINDICATIONSMetaxalone is contraindicated in patients with: Known hypersensitivity to any component of metaxalone. Known tendency to drug induced, hemolytic, or other anemias. Severe renal or hepatic ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serotonin Syndrome - Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of metaxalone (within the recommended dosage range) and ...
-
6 ADVERSE REACTIONSThe following adverse reactions associated with the use of metaxalone were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a ...
-
7 DRUG INTERACTIONS7.1 Serotonergic Drugs - If concomitant use of metaxalone and another serotoneric drug is warranted, carefully observe the patient, particularly during treatment initiation and dosage ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on metaxalone use in pregnant patients to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse ...
-
10 OVERDOSAGEClinical Presentation of Metaxalone Overdose - Deaths by deliberate or accidental overdose have occurred with metaxalone, particularly in combination with other CNS depressants (including ...
-
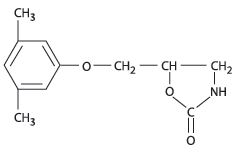
11 DESCRIPTIONMetaxalone Tablets, USP contain 800 mg of metaxalone and the following inactive ingredients: carboxymethylcellulose sodium, alginic acid, stearic acid, hydrogenated castor oil, magnesium stearate ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metaxalone’s mechanism of action has not been fully characterized, but may be related to its sedative properties. Metaxalone has no direct action on the contractile ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies to evaluate the carcinogenic potential of metaxalone have not been conducted. Studies to evaluate the mutagenic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMetaxalone Tablets, USP are available as 800 mg pink, capsule shaped, scored tablets, debossed “LCI" on one side and "14" bisect "35" on the other side. Available in - NDC: 70518-0771-00 - NDC ...
-
17 PATIENT COUNSELING INFORMATIONSerotonin Syndrome - Inform patients that metaxalone could cause a rare but potentially life-threatening condition called serotonin syndrome. Warn patients of the symptoms of serotonin syndrome ...
-
PRINCIPAL DISPLAY PANELDRUG: Metaxalone - GENERIC: Metaxalone - DOSAGE: TABLET - ADMINSTRATION: ORAL - NDC: 70518-0771-0 - NDC: 70518-0771-1 - NDC: 70518-0771-2 - NDC: 70518-0771-3 - NDC: 70518-0771-4 - COLOR: pink - SHAPE: OVAL - SCORE: Two ...
-
INGREDIENTS AND APPEARANCEProduct Information