Label: VISIPAQUE- iodixanol injection, solution
- NDC Code(s): 0407-2222-01, 0407-2222-02, 0407-2222-03, 0407-2222-06, view more
- Packager: GE Healthcare Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VISIPAQUE safely and effectively. See full prescribing information for VISIPAQUE. VISIPAQUE (iodixanol) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: NOT FOR INTRATHECAL USE
Inadvertent intrathecal administration may cause death, convulsions/seizures, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, rhabdomyolysis, hyperthermia, and brain edema [see Contraindications (4) and Adverse Reactions (5.1)].
Close -
1 INDICATIONS AND USAGEVISIPAQUE is indicated in for: 1.1 Intra-arterial Procedures - Adult and pediatric patients 12 years of age and older - (270 and 320 mg Iodine/mL) intra-arterial digital subtraction angiography ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Instructions - VISIPAQUE is for intravascular use only [see Boxed Warning, Contraindications (4), and Warnings and Precautions (5.1)] Use sterile ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: Non-ionic, isotonic, water-soluble, sterile, pyrogen-free, colorless to pale yellow solution in the following strengths: 270 mg of organically bound iodine per mL (550 mg Iodixanol per ...
-
4 CONTRAINDICATIONSVISIPAQUE is contraindicated for intrathecal use [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Risks Associated with Inadvertent Intrathecal Administration - VISIPAQUE is for intravascular use only and is contraindicated for intrathecal use [see Contraindications (4) and Dosage and ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Risks Associated with Inadvertent Intrathecal Administration [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Drug-Drug Interactions - Metformin - In patients with renal impairment, metformin can cause lactic acidosis. Iodinated contrast agents appear to increase the risk of metformin-induced ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data with iodixanol use in pregnant women to inform any drug-associated risks. In animal reproduction studies, no developmental toxicity occurred ...
-
10 OVERDOSAGEThe adverse effects of overdosage of any contrast agent may be life-threatening and affect mainly the pulmonary and cardiovascular systems. Treatment of an overdosage is directed toward the ...
-
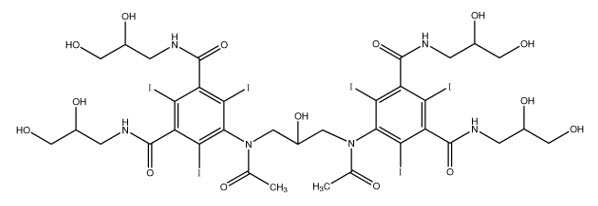
11 DESCRIPTION11.1 Chemical Characteristics - VISIPAQUE (iodixanol) injection is a dimeric, iso-osmolar, nonionic, water-soluble, radiographic contrast medium for intravascular (intravenous and intra-arterial ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Intravascular injection of iodixanol opacifies vessels in the path of flow of the contrast agent, permitting visualization of internal structures. In imaging of the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed with iodixanol to evaluate carcinogenic potential. Iodixanol was not genotoxic in a ...
-
14 CLINICAL STUDIESVISIPAQUE was studied in 1244 adult patients. Approximately one-half (590) of the VISIPAQUE patients were 60 years of age or older; the mean age was 56 years (range 18 to 90). A total of patients ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - VISIPAQUE injection is a ready-to-use sterile, pyrogen-free, preservative free, colorless to pale yellow solution available in two (2) strengths. It is supplied in the ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity Reactions - Advise the patient concerning the risk of hypersensitivity reactions that can occur both during and after VISIPAQUE administration. Advise the patient to report any ...
-
SPL UNCLASSIFIED SECTIONDistributed by GE Healthcare Inc., Marlborough, MA 01752 U.S.A. Product of Norwegian Origin. VISIPAQUE is a trademark of GE HealthCare or one of its subsidiaries. GE is a trademark of General ...
-
PRINCIPAL DISPLAY PANEL - 270 mgI/mL Bottle Box LabelGE Healthcare - V-552 - Contains - 10 x 100 mL - Bottles - NDC 0407-2222-17 - VISIPAQUE™ (iodixanol) Injection - 270 mgI/mL - NOT FOR INTRATHECAL USE - in +PLUSPAK™ (polymer bottle) Single-Dose Bottle. Sterile ...
-
PRINCIPAL DISPLAY PANEL - 320 mgI/mL Bottle Box LabelGE Healthcare - V-562 - Contains - 10 x 100 mL - Bottles - NDC 0407-2223-17 - VISIPAQUE™ (iodixanol) Injection - 320 mg Iodine/mL - For Intra-arterial and - Intravenous Use Only - in +PLUSPAK™ (polymer ...
-
INGREDIENTS AND APPEARANCEProduct Information