Label: DOXYCYCLINE capsule
- NDC Code(s): 51407-678-01
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 64380-179
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of doxycycline capsules and other antibacterial drugs, doxycycline capsules should be used only to treat or ...
-
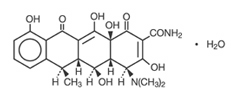
DESCRIPTIONDoxycycline Capsules USP is a broad-spectrum antibacterial synthetically derived from oxytetracycline. Doxycycline 150 mg, 100 mg and 50 mg capsules contain doxycycline monohydrate equivalent to ...
-
CLINICAL PHARMACOLOGYTetracyclines are readily absorbed and are bound to plasma proteins in varying degrees. They are concentrated by the liver in the bile and excreted in the urine and feces at high concentrations ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain effectiveness of doxycycline capsules and other antibacterial drugs, doxycycline capsules should be used only to treat or prevent ...
-
CONTRAINDICATIONSThis drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
WARNINGSThe use of drugs of the tetracycline class, including doxycycline, during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration ...
-
PRECAUTIONSGeneral: As with other antibacterial preparations, use of this drug may result in overgrowth of non-susceptible organisms, including fungi. If superinfection occurs, doxycycline capsules should ...
-
ADVERSE REACTIONSDue to oral doxycycline's virtually complete absorption, side effects to the lower bowel, particularly diarrhea, have been infrequent. The following adverse reactions have been observed in ...
-
OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Dialysis does not alter serum half-life, and it would not be of benefit in treating cases ...
-
DOSAGE AND ADMINISTRATIONTHE USUAL DOSAGE AND FREQUENCY OF ADMINISTRATION OF DOXYCYCLINE DIFFERS FROM THAT OF THE OTHER TETRACYCLINES. EXCEEDING THE RECOMMENDED DOSAGE MAY RESULT IN AN INCREASED INCIDENCE OF SIDE ...
-
HOW SUPPLIEDDoxycycline Capsules USP 50 mg have a buff opaque cap printed "par 726" in brown ink/white opaque body printed "par 726" in brown ink. Each capsule contains doxycycline monohydrate equivalent to ...
-
ANIMAL PHARMACOLOGY AND ANIMAL TOXICOLOGYHyperpigmentation of the thyroid has been produced by members of the tetracycline class in the following species: in rats by oxytetracycline, doxycycline, tetracycline PO - 4, and methacycline ...
-
REFERENCESFriedman JM and Polifka JE. Teratogenic Effects of Drugs. A Resource for Clinicians (TERIS). Baltimore, MD: The Johns Hopkins University Press: 2000: 149-195. Cziezel AE and Rockenbauer M ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information