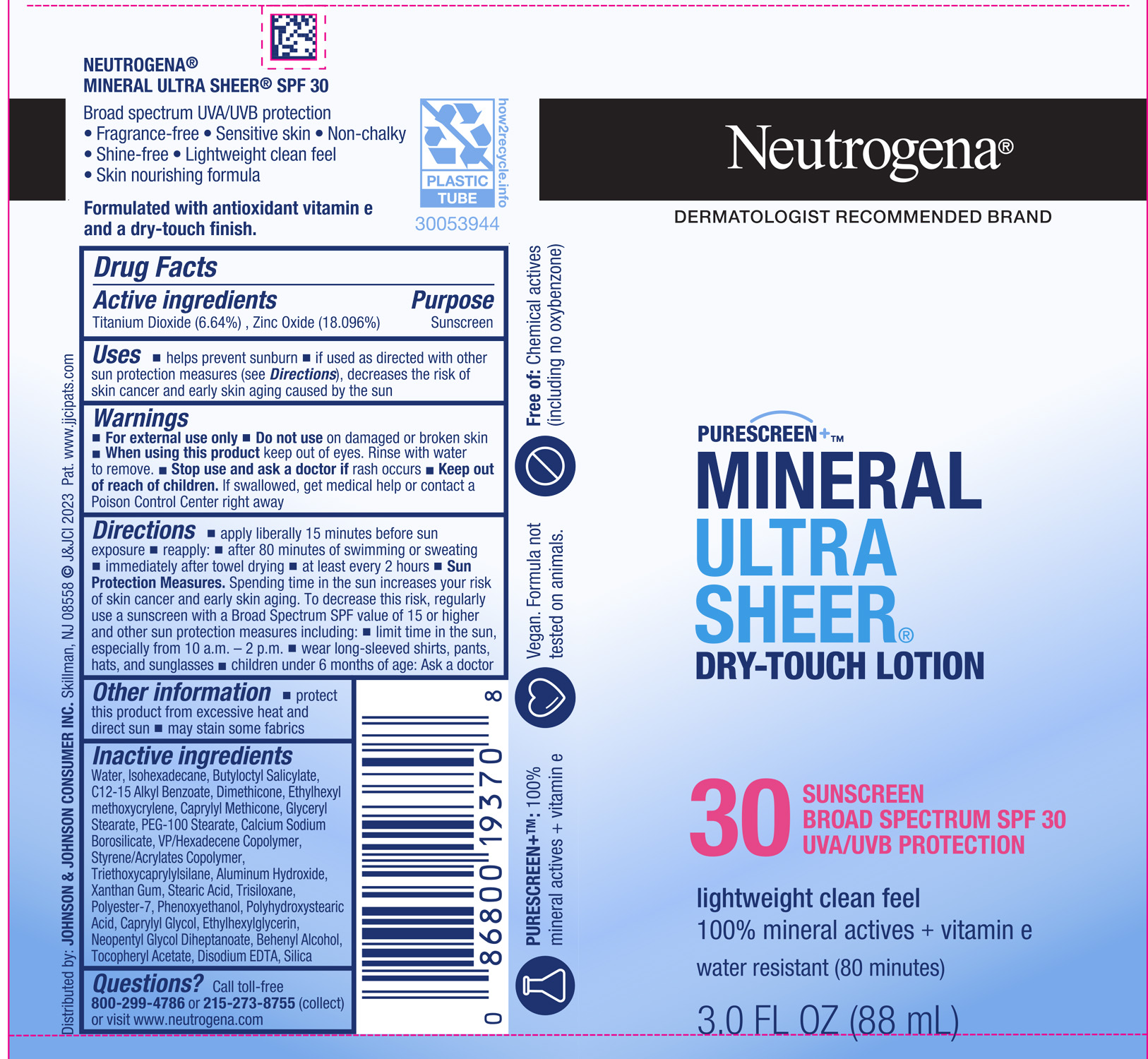
Label: NEUTROGENA MINERAL ULTRA SHEER DRY TOUCH SPF 30- titanium dioxide, zinc oxide lotion
- NDC Code(s): 69968-0776-1, 69968-0776-3
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Water, Isohexadecane, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Dimethicone, Ethylhexyl methoxycrylene, Caprylyl Methicone, Glyceryl Stearate, PEG-100 Stearate, Calcium Sodium Borosilicate, VP/Hexadecene Copolymer, Styrene/Acrylates Copolymer, Triethoxycaprylylsilane, Aluminum Hydroxide, Xanthan Gum, Stearic Acid, Trisiloxane, Polyester-7, Phenoxyethanol, Polyhydroxystearic Acid, Caprylyl Glycol, Ethylhexylglycerin, Neopentyl Glycol Diheptanoate, Behenyl Alcohol, Tocopheryl Acetate, Disodium EDTA, Silica
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 88 mL Tube Label
-
INGREDIENTS AND APPEARANCE
NEUTROGENA MINERAL ULTRA SHEER DRY TOUCH SPF 30
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0776 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 180 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 66 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOHEXADECANE (UNII: 918X1OUF1E) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) DOCOSANOL (UNII: 9G1OE216XY) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) DIMETHICONE (UNII: 92RU3N3Y1O) TRISILOXANE (UNII: 9G1ZW13R0G) CALCIUM SODIUM BOROSILICATE (UNII: 4MM76N4WMY) PEG-100 STEARATE (UNII: YD01N1999R) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) STEARIC ACID (UNII: 4ELV7Z65AP) POLYESTER-7 (UNII: 0841698D2F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0776-3 88 mL in 1 TUBE; Type 0: Not a Combination Product 12/06/2022 2 NDC:69968-0776-1 12 in 1 TRAY 12/06/2022 2 14 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/06/2022 Labeler - Kenvue Brands LLC (118772437)