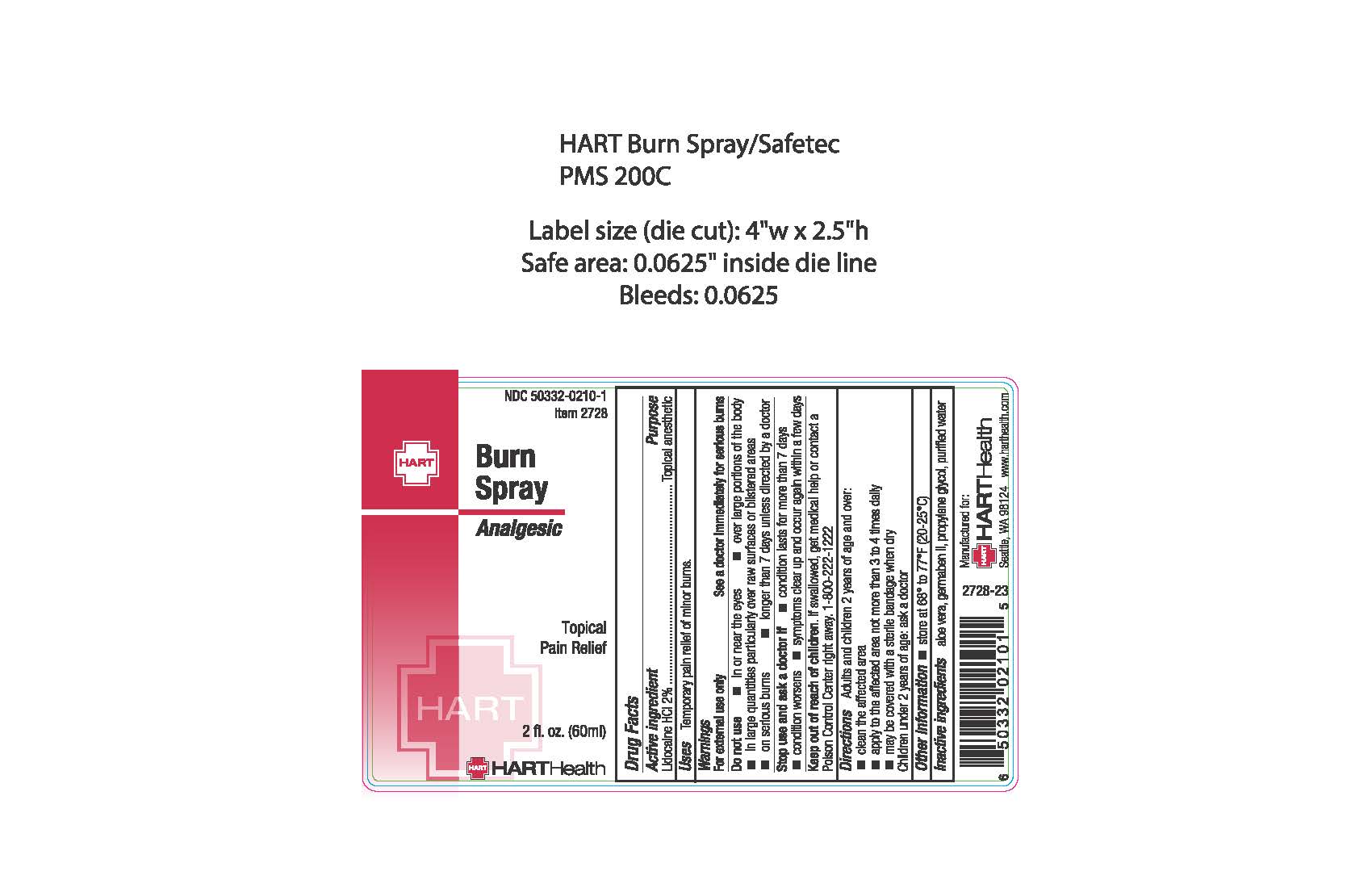
Label: BURN- lidocaine spray
- NDC Code(s): 50332-0210-1
- Packager: HART Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
See a doctor immediately or serious burns
Do not use
- in or near the eyes
- over large portions of the body
- in large quantities particularly over raw surfaces or blistered areas
- on serious burns
- longer than 7 days unless directed by a doctor
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BURN
lidocaine sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50332-0210 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA WHOLE (UNII: KIZ4X2EHYX) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50332-0210-1 60 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2024 Labeler - HART Health (069560969) Registrant - HART Health (069560969)