NEMLUVIO® [Nem LOO vee oh]
(nemolizumab-ilto)
for injection, for subcutaneous use
-
This Instructions for Use contains information on how to inject NEMLUVIO.
Read and understand these ...
NEMLUVIO® [Nem LOO vee oh]
(nemolizumab-ilto)
for injection, for subcutaneous use
This Instructions for Use contains information on how to inject NEMLUVIO.
Read and understand these instructions before using the NEMLUVIO pen.
Do not inject yourself or someone else until you have been instructed how to inject NEMLUVIO.
Your healthcare provider will instruct you or your caregiver how to prepare and inject a dose of NEMLUVIO before you try to do it yourself the first time. Call your healthcare provider if you have any questions.
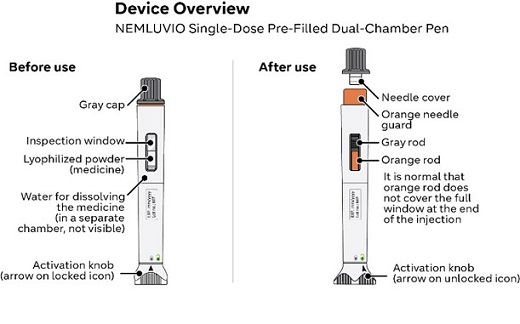
NEMLUVIO is supplied as a single-dose pen (called NEMLUVIO pen or pen in these instructions). It contains medicine (30 mg of lyophilized powder) in one chamber and water for dissolving the medicine in the other chamber. Before you can inject it, you must mix the lyophilized powder with the water for dissolving the medicine.
Important Information
What you need to know before using the NEMLUVIO pen:
- Read all the instructions carefully before using the NEMLUVIO pen.
-
Mark your calendar ahead of time to remember when to take NEMLUVIO.
- Follow all steps exactly as described. This makes sure that you get the correct dose of medicine.
- Make sure that the lyophilized powder is completely dissolved before injecting (Step 9).
- After dissolving, proceed right away with the injection to avoid any contamination or break down of medicine (degradation).
-
Do not use the NEMLUVIO pen if it has been dropped on a hard surface or is damaged, cracked or broken.
- In some cases, your healthcare provider may prescribe 2 pens for a full dose. Use 1 NEMLUVIO pen after the other.
- To reduce the risk of accidental needle stick injury, each NEMLUVIO pen has an orange needle guard. After injecting the medicine, as you lift the pen from your skin, the orange needle guard locks into place to cover the needle (Step 16).
- When preparing the pen for injection, do not pull the gray cap. Instead, twist the gray cap until the orange needle guard pops up. Then, gently pull the cap off the orange needle guard (Step 12).
- Throw away (dispose of) the used NEMLUVIO pen right away after use in a sharps disposal container. See Section C: Throwing away (Disposing of) NEMLUVIO below.
Storage Information:
- Store the NEMLUVIO pen in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date.
- Store the NEMLUVIO pen in the original carton to protect it from light.
- The NEMLUVIO pen can be stored at room temperature up to 77°F (25°C) for a single period up to 90 days. Thow away (dispose of) the NEMLUVIO pen after the expiration date and any NEMLUVIO pen that has been left at room temperature longer than 90 days.
- After the NEMLUVIO pen lyophilized powder and water for injection are mixed (reconstituted), the NEMLUVIO pen must be used within 4 hours or thrown away (discarded).
-
Do not heat or put the NEMLUVIO pen into direct sunlight.
-
Do not freeze the NEMLUVIO pen.
- If the NEMLUVIO pen was heated or frozen, throw away (dispose of it).
Keep the NEMLUVIO pen and all medicines out of the reach of children.
Traveling Information:
- Generally you are allowed to carry pens with you on an airplane. Be sure to carry the NEMLUVIO pens with you in your carry-on luggage
If you have any other questions refer to the Frequently asked questions (FAQs) on the back of this leaflet.
A. Preparing to inject NEMLUVIO
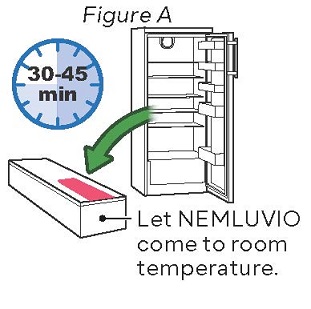
Step 1: Let NEMLUVIO reach room temperature
Injecting cold medicine might result in pain at the injection site.
Take the NEMLUVIO carton out of the refrigerator and let it come to room temperature for 30 to 45 minutes before starting Step 2 (see Figure A).
Do not:
- warm the pen with any heat source (such as microwave or direct sunlight). This might damage NEMLUVIO.
- directly expose the pen to liquids.
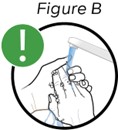
Step 2: Wash your hands
a. To avoid contamination and infection, wash your hands with soap (see Figure B).
b. Dry them properly.
Step 3: Gather Supplies (see Figure C)
a. Remove the pen from the carton.
b. Gather the following supplies on a clean, flat and well-lit surface:
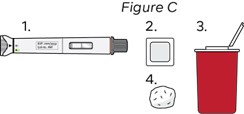
- Pen
- Alcohol wipes*
- Sharps disposal container*
- Gauze pads or cotton balls*
*Items not included in the carton.
Note: In some cases, your healthcare provider may prescribe 2 pens. Use 1 pen after the other.
Step 4: Check the NEMLUVIO pen for the following:
a. Expiration date has not passed.
b. The lyophilized powder is white and not dissolved (see Figure D).
c. The pen has not been dropped and is not damaged or cracked.
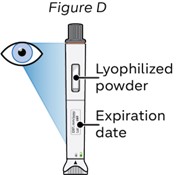
Do not use the pen, unless all conditions above are met.
If any condition is not met, call: 1-866-735-4137.
Throw away (dispose of) the pen and use a new one (see Section C: Throwing away (disposing of) NEMLUVIO).
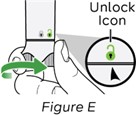
Step 5: Activate the NEMLUVIO pen
Hold the pen upright and turn activation knob to the right until it stops (see Figure E).
This starts the process of transferring water to the powder chamber.
Step 6: Wait until the gray rod stops moving
Watch inspection window until gray rod has stopped moving (see Figure F).
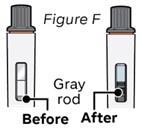
Do not shake the pen. Shaking the pen before the gray rod has completely stopped can affect the medicine dose.
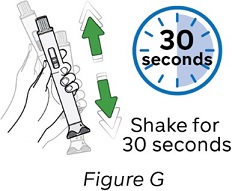
Step 7: Dissolve the medicine
When the gray rod has completely stopped, shake the pen up and down for 30 seconds (see Figure G).
Step 8: Wait 5 minutes for bubbles to decrease
Wait for bubbles to decrease and the lyophilized powder to dissolve completely. This will take about 5 minutes (see Figure H).
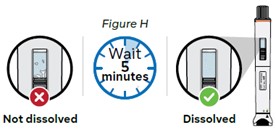
Note: If the medicine has not dissolved completely, shake the pen up and down again for 30 seconds and then wait 5 minutes.
Note: It is normal for a small foam layer or a few small air bubbles to remain in the dissolved medicine.
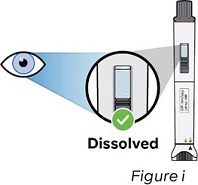
Step 9: Check the medicine in the inspection window
Check to see if the dissolved medicine:
- is clear and colorless to slightly yellow,
- does not contain particles (see Figure i).
Do not use the pen if the dissolved medicine is cloudy or contains any particles.
Throw away (dispose of) the pen and use a new one (see Section C: Throwing away (Disposing of) NEMLUVIO).
Note: After the medicine has dissolved, it must be used within 4 hours. During this time, it should be kept at room temperature (up to 77°F (25°C)). If you have not used it within 4 hours, throw it away (dispose of it).
B. Injecting NEMLUVIO
Step 10: Select one injection site (see Figure J)
Note: When using a second pen, select a different injection site at least 1 inch away from the first injection site.
Select the injection site using the following chart:
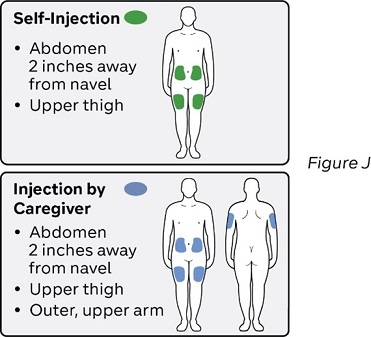
Where not to inject:
- near your waistline or about 2 inches around the navel.
- into tender, bruised, red skin, or areas with scars or stretch marks
- twice into the same site (example, within, 1 inch).
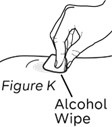
Step 11: Clean the injection site (see Figure K)
a. Always use a new alcohol wipe to clean the injection site.
This avoids contamination and infection.
b. Let the skin air dry.
Do not:
- touch the injection site after cleaning.
- fan, or blow air on the cleaned injection site.
- reuse the alcohol wipe.
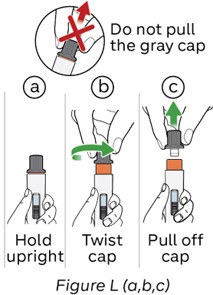
Step 12: Twist the gray cap
Do not:
- pull the gray cap when twisting to avoid damaging the device.
- touch the orange needle guard.
-
Hold the pen upright (see Figure L, a)
- Twist the gray cap until the orange needle guard pops up (see Figure L, b).
- Gently pull the cap off the orange needle guard (see Figure L, c)
- After cap removal, please throw away (dispose of) the cap in a sharps disposal container (see Step 17).
Note: If the cap cannot be removed, refer back to Step 5 and make sure the activation knob is turned completely to the right until it stops.
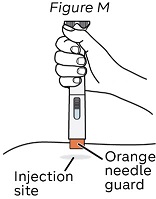
Step 13: Place the NEMLUVIO pen
Read Steps 13-16 before starting Step 13.
Note: Always inject the way your healthcare provider showed you.
Place the pen on the injection site vertically so that the orange needle guard is flat against the skin (see Figure M).
Note: Make sure you can easily see the inspection window during injection.
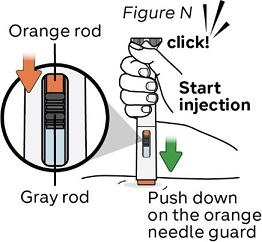
Step 14: Start injection and hold the NEMLUVIO pen on the skin
Gently push the pen down until the orange needle guard is completely pushed in.
The injection starts right away with a click (see Figure N).
The orange rod will begin to move down the injection window.
Do not lift the pen yet and keep pushing down.
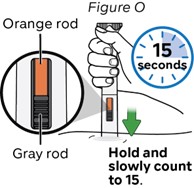
Step 15: Inject for 15 seconds
Hold and slowly count to 15.
Check inspection window to make sure the orange rod and the gray rod have stopped (see Figure O).
This means the injection has been completed.
Note: It is normal that the orange rod does not cover the whole inspection window at the end of injection.
Do not lift the pen until the orange rod and gray rod have stopped moving.
If the orange rod is not visible please call: 1-866-735-4137. Throw away (dispose of) the pen and use a new one (see Section C for disposal details).
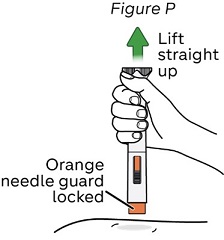
Step 16: Lift the NEMLUVIO pen up
a. Lift the pen straight up from your skin.
The orange needle guard locks into place to cover the needle (see Figure P).
b. If there is bleeding, press a cotton ball or gauze over the injection site.
Do not rub the injection site.
C. Throwing away (disposing of) NEMLUVIO
Step 17: Throw away (dispose of) NEMLUVIO in a sharps disposal container
Avoid contact with the needle.
Throw away (dispose of) the used pen and the gray cap in an FDA-cleared sharps disposal container right away after use (see Figure Q).
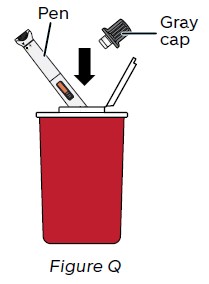
Do not:
- recap the pen after use,
- dispose of the NEMLUVIO pen and cap in your household trash,
- dispose of your used sharps disposal container in your household trash unless your community guidelines permit this,
- recycle your used sharps disposal container.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should dispose of used pens.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
http://www.fda.gov/safesharpsdisposal
FAQs (Frequently asked questions)
Manufactured by:
Galderma Laboratories, L.P.
Dallas, TX 75201
This Instructions for Use has been approved by the U.S. Food and Drug Administration
U.S. License No. 2289
Issued: 05/2025
P502292-X
Close