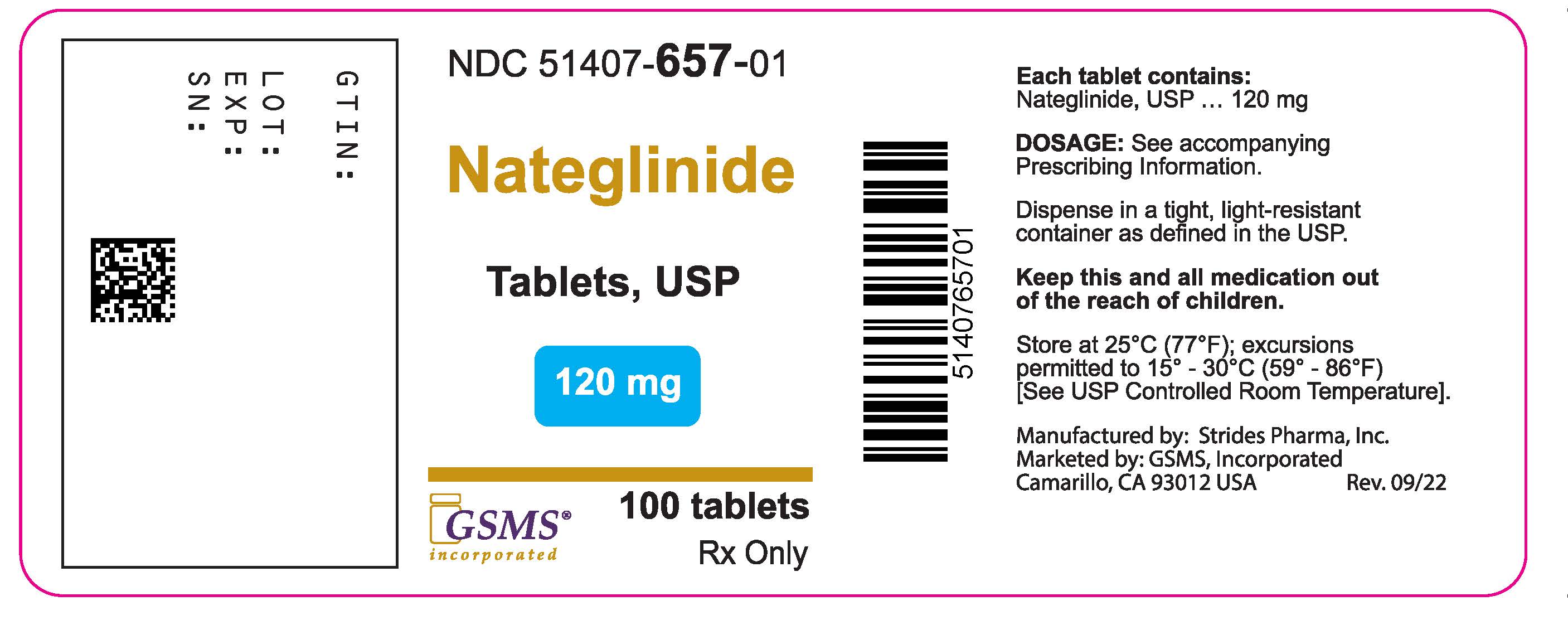
Label: NATEGLINIDE tablet
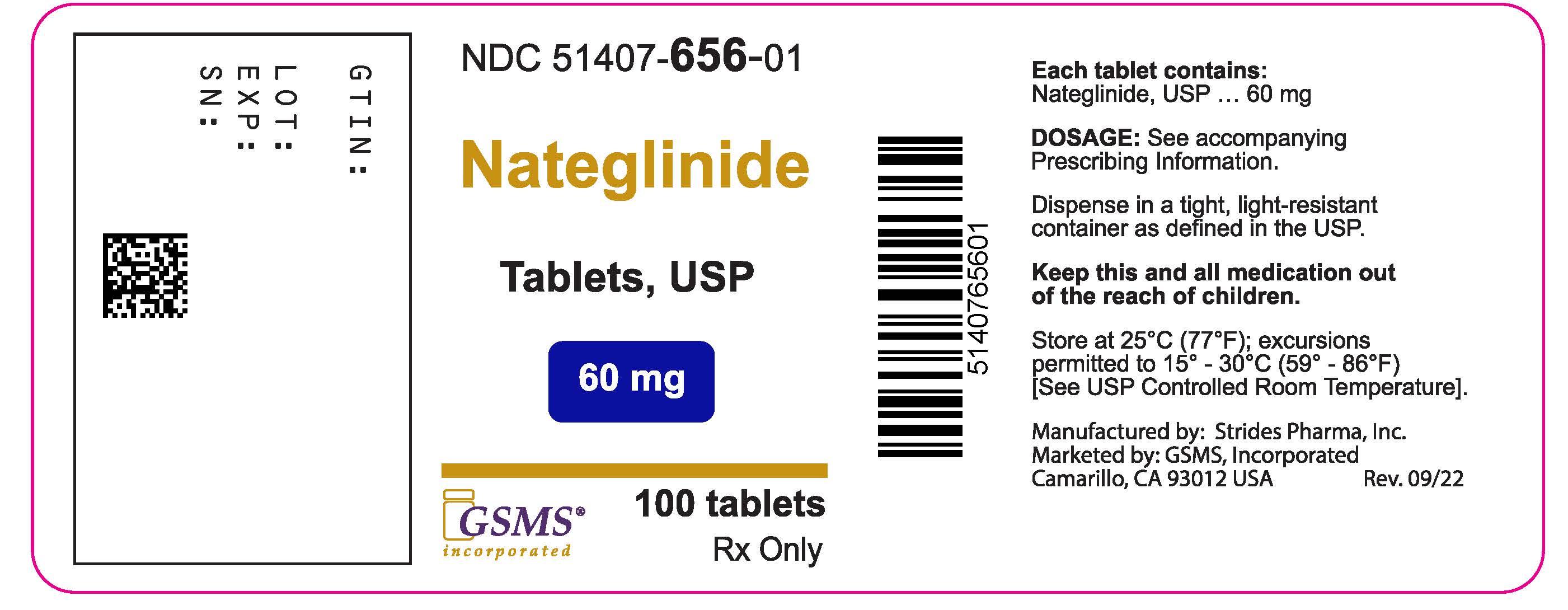
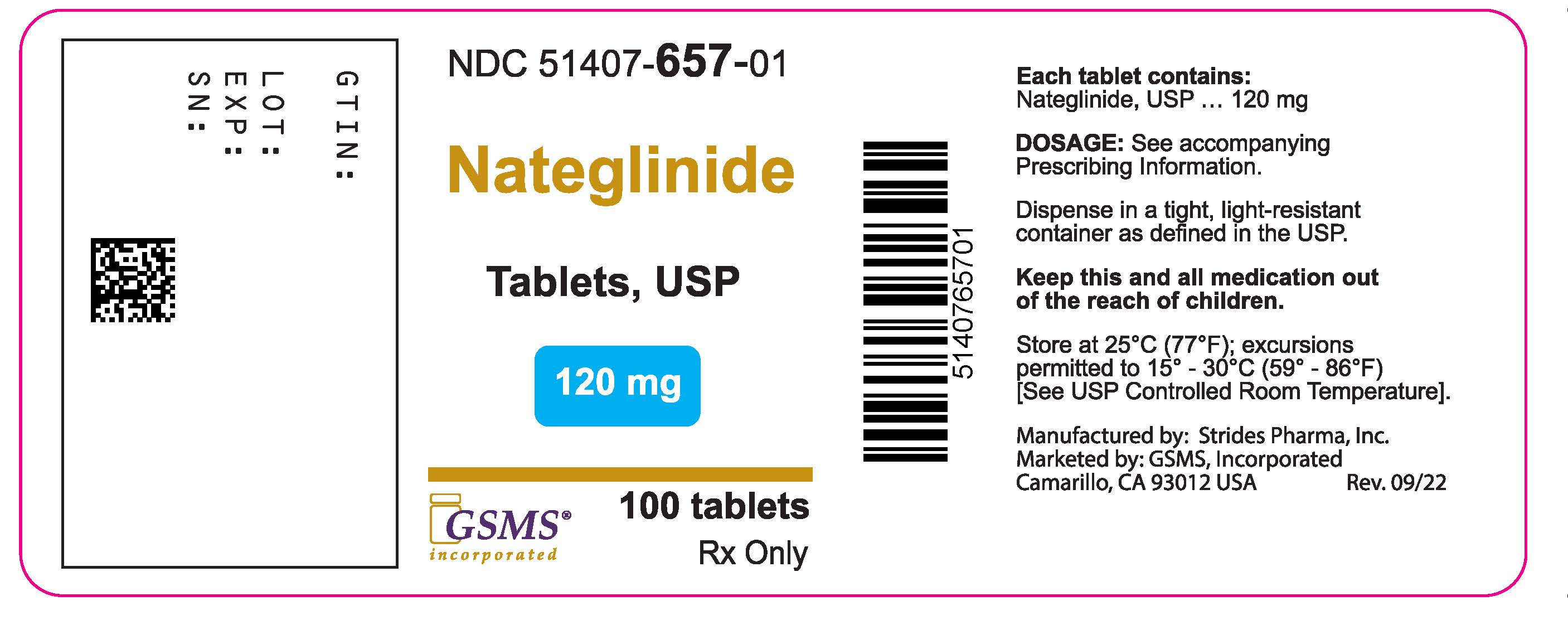
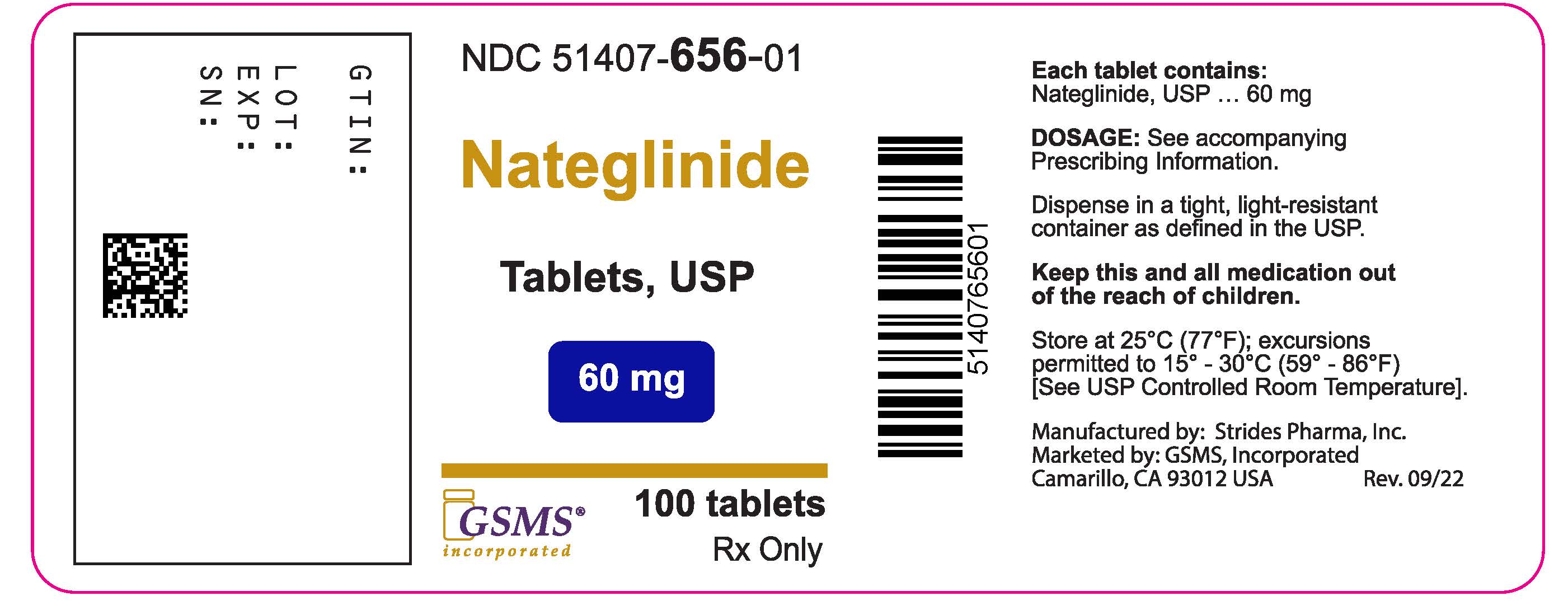
- NDC Code(s): 51407-656-01, 51407-657-01
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 64380-167, 64380-168
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONNATEGLINIDE TABLETS. These highlights do not include all the information needed to use NATEGLINIDE TABLETS safely and effectively. See full prescribing information for NATEGLINIDE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENateglinide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Limitations of Use: Nateglinide should not be used in patients ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose of nateglinide is 120 mg orally three times daily before meals. The recommended dose of nateglinide is 60 mg orally three times daily before meals in patients who are near ...
-
3 DOSAGE FORMS AND STRENGTHS60 mg tablets: Pink color coated, round biconvex, beveled edge tablet debossed with "P 984" on one side and plain on the other side - 120 mg tablets: Orange color coated, oval shaped biconvex ...
-
4 CONTRAINDICATIONSNateglinide tablets are contraindicated in patients with a history of hypersensitivity to nateglinide or its inactive ingredients.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypoglycemia - All glinides, including nateglinide, can cause hypoglycemia - [see Adverse Reactions (6.1)]. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death ...
-
6 ADVERSE REACTIONSThe following serious adverse reaction is also described elsewhere in the labeling: Hypoglycemia - [see Warnings and Precautions (5.1)] 6.1 Clinical Trials Experience - Because clinical ...
-
7 DRUG INTERACTIONSTable 2 includes a list of drugs with clinically important drug interactions when concomitantly administered or withdrawn with nateglinide and instructions for managing or preventing ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The available data from published literature and the applicant's pharmacovigilance with use of nateglinide in pregnant women are insufficient to identify a ...
-
10 OVERDOSAGEThere have been no instances of overdose with nateglinide in clinical trials. However, an overdose may result in an exaggerated glucose-lowering effect with the development of hypoglycemic ...
-
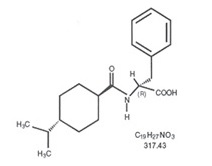
11 DESCRIPTIONNateglinide Tablets, USP are an oral blood glucose-lowering drug of the glinide class. Nateglinide, (-)-N-[(trans-4-isopropylcyclohexane)carbonyl]-D-phenylalanine, is structurally unrelated to ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nateglinide lowers blood glucose levels by stimulating insulin secretion from the pancreas. This action is dependent upon functioning beta-cells in the pancreatic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity:Nateglinide did not increase tumors in two year carcinogenicity studies conducted in mice and rats. Oral doses of ...
-
14 CLINICAL STUDIES14.1 Monotherapy - In a 24-week, double-blind, placebo-controlled study, patients with type 2 diabetes were randomized to receive either nateglinide (60 mg or 120 mg three times daily before ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Nateglinide Tablets, USP are supplied in the following package and dose strength forms: 60 mg - Pink color coated, round biconvex, beveled edge tablet debossed with "P 984" on one ...
-
17 PATIENT COUNSELING INFORMATIONAdministration - Instruct patients to take nateglinide 1 to 30 minutes before meals. Instruct patients that skip meals to skip their dose of nateglinide - [see Dosage and Administration (2)] ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANELPackage Label - Rx Only - NDC 51407-656-01 - Nateglinide Tablets - 60 mg - 100 Tablets.
-
PACKAGE LABEL PRINCIPAL DISPLAY PANELPackage Label - Rx Only - NDC 51407-657-01 - Nateglinide Tablets - 120 mg - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information