Label: OMEPRAZOLE AND SODIUM BICARBONATE capsule
- NDC Code(s): 68382-503-04, 68382-503-17, 68382-503-67
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
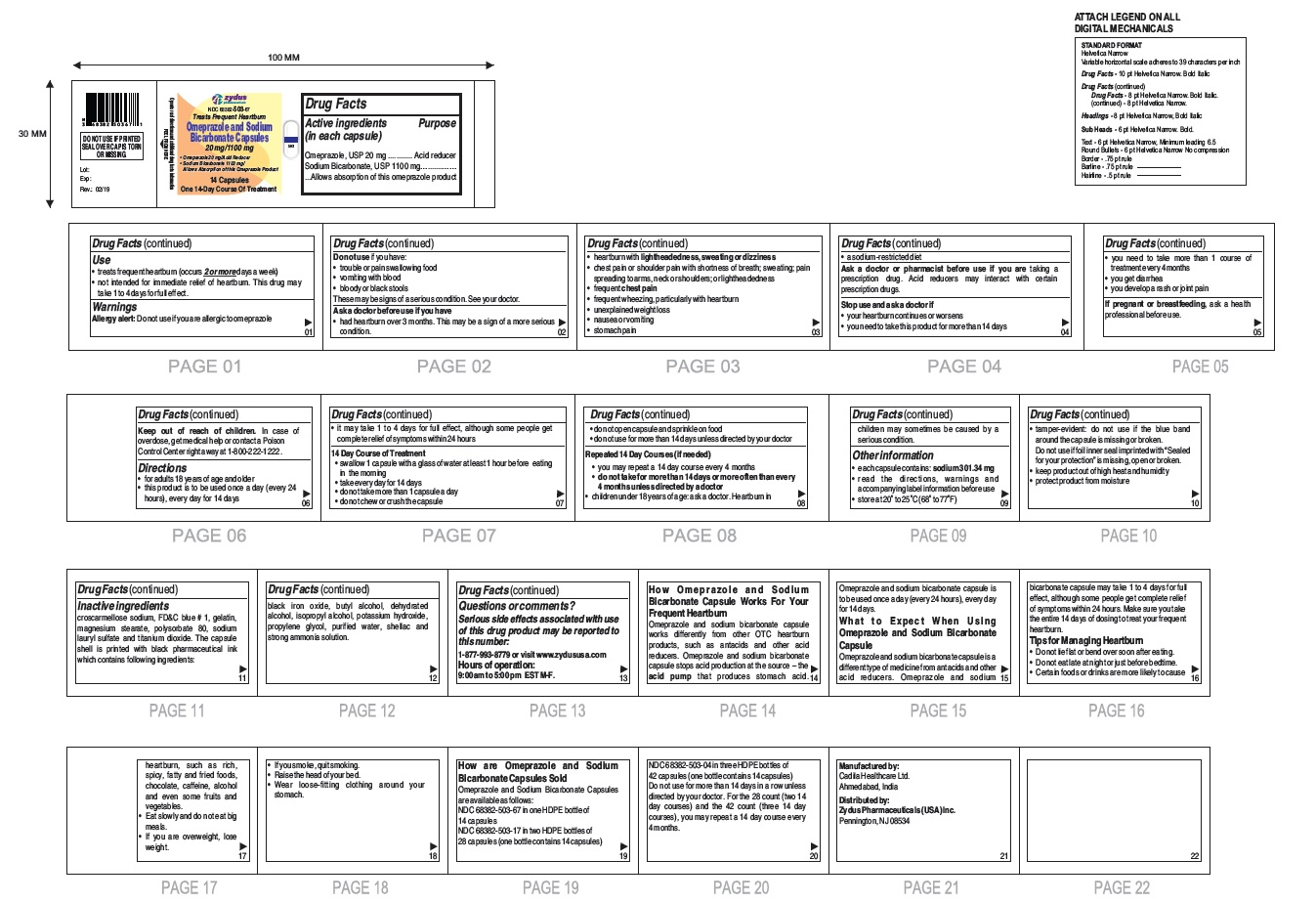
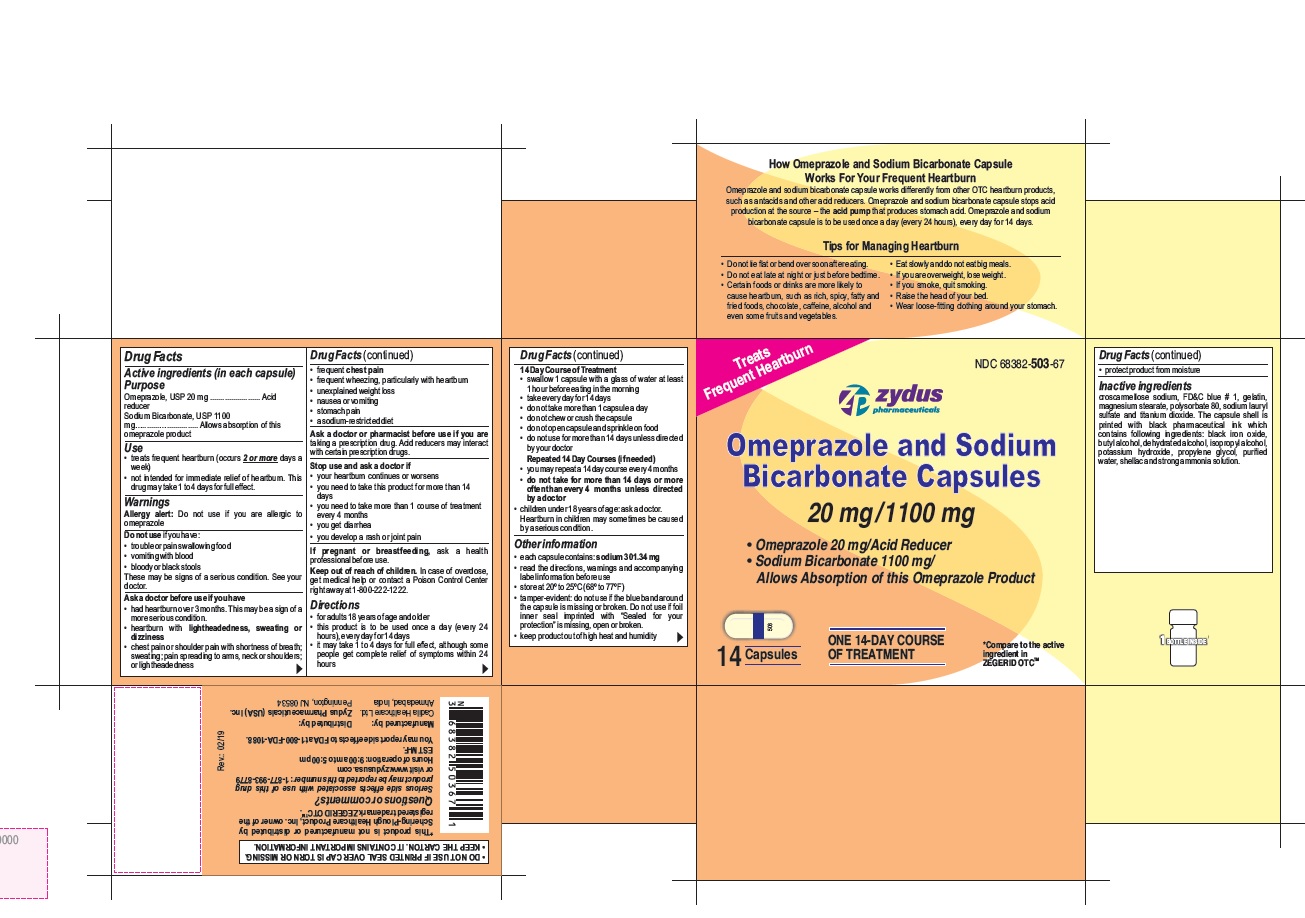
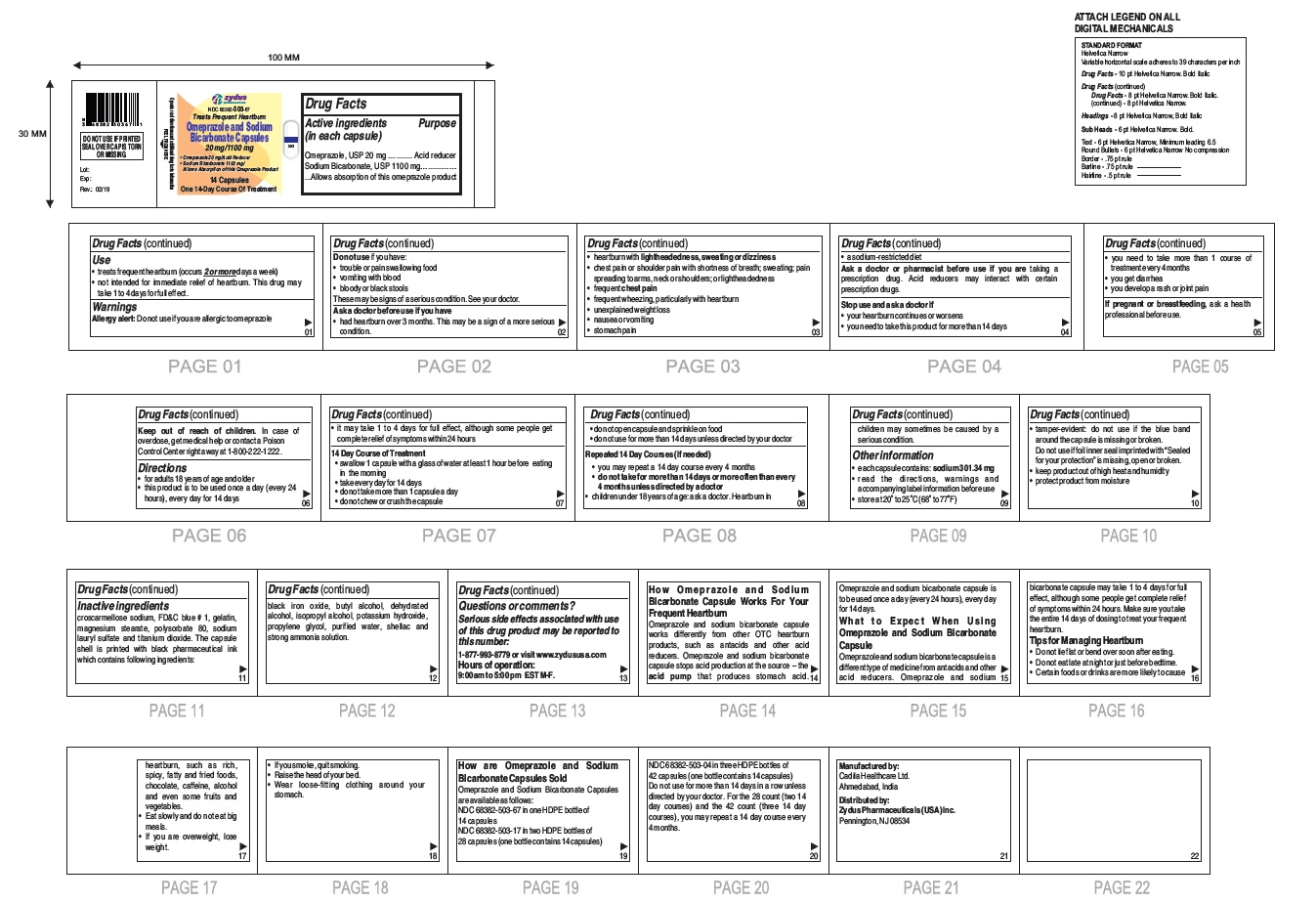
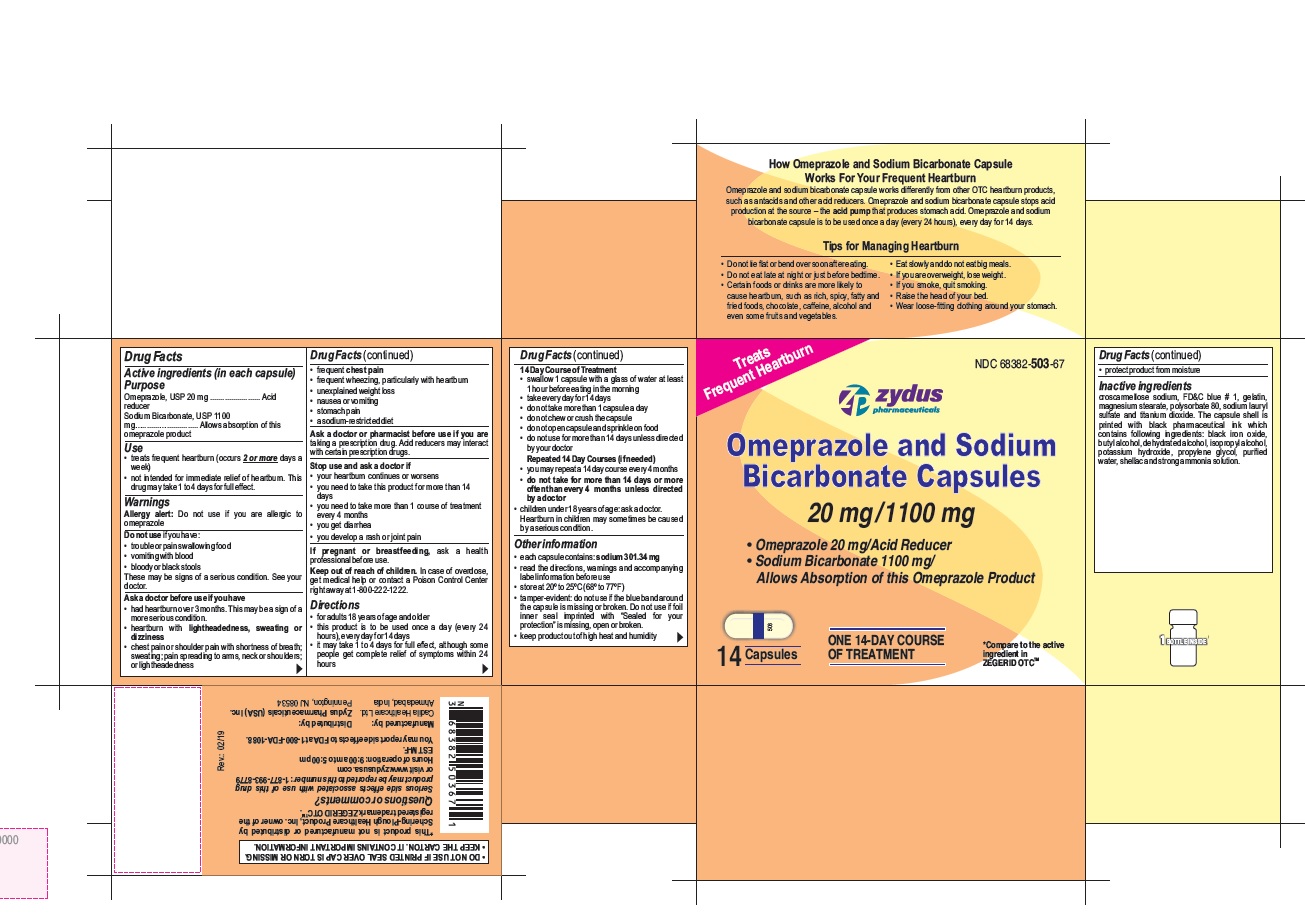
- Active ingredients (in each capsule)
- Purpose
- Use
- Warnings
- Do not use if you have:
-
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- a sodium-restricted diet
- Ask a doctor or pharmacist before use if you are taking
- Stop use and ask a doctor if
- SPL UNCLASSIFIED SECTION
- Keep out of reach of children.
- Directions
-
14 Day Course of Treatment
- swallow 1 capsule with a glass of water at least 1 hour before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- do not chew or crush the capsule
- do not open capsule and sprinkle on food
- do not use for more than 14 days unless directed by your doctor
Repeated 14 Day Courses (if needed)
- you may repeat a 14 day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
• children under 18 years of age: ask a doctor.
Heartburn in children may sometimes be caused by a serious condition.
-
Other information
- each capsule contains: sodium 301.34 mg
- read the directions, warnings and accompanying label information before use
- store at 20○ to 25○C (68○ to 77○F)
- tamper-evident: do not use if the blue band around the capsule is missing or broken. Do not use if foil inner seal imprinted with "Sealed for your protection" is missing, open or broken.
- keep product out of high heat and humidity
- protect product from moisture
-
Inactive ingredient
croscarmellose sodium, FD&C blue # 1, gelatin, magnesium stearate, polysorbate 80, sodium lauryl sulfate and titanium dioxide. The capsule shell is printed with black pharmaceutical ink which contains following ingredients: black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac and strong ammonia solution.
-
SPL UNCLASSIFIED SECTION
Serious side effects associated with use of this drug product may be reported to this number: 1-877-993-8779 or visit www.zydususa.com
Hours of operation:
9:00 am to 5:00 pm
EST M-F.
You may report side effects to FDA at 1-800-FDA-1088.
How Omeprazole and Sodium Bicarbonate Capsule Works For Your Frequent Heartburn
Omeprazole and sodium bicarbonate capsule works differently from other OTC heartburn products, such as antacids and other acid reducers. Omeprazole and sodium bicarbonate capsule stops acid production at the source – the acid pump that produces stomach acid. Omeprazole and sodium bicarbonate capsule is to be used once a day (every 24 hours), every day for 14 days.
What to Expect When Using Omeprazole and Sodium Bicarbonate Capsule
Omeprazole and sodium bicarbonate capsule is a different type of medicine from antacids and other acid reducers. Omeprazole and sodium bicarbonate capsule may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours. Make sure you take the entire 14 days of dosing to treat your frequent heartburn.
Tips for Managing Heartburn
- Do not lie flat or bend over soon after eating.
- Do not eat late at night or just before bedtime.
- Certain foods or drinks are more likely to cause heartburn, such as rich, spicy, fatty and fried foods, chocolate, caffeine, alcohol and even some fruits and vegetables.
- Eat slowly and do not eat big meals.
- If you are overweight, lose weight.
- If you smoke, quit smoking.
- Raise the head of your bed.
- Wear loose-fitting clothing around your stomach.
How are Omeprazole and Sodium Bicarbonate Capsules Sold
Omeprazole and Sodium Bicarbonate Capsules, 20 mg/1100 mg are white to off-white fine powder filled in hard gelatin capsule shells of size '00' having an opaque white cap and an opaque white body printed with '503' on body in black ink and a blue tamper evident band and are available as follows:
NDC 68382-503-67 in one HDPE bottle of 14 capsules
NDC 68382-503-17 in two HDPE bottles of 28 capsules (one bottle contains 14 capsules)
NDC 68382-503-04 in three HDPE bottles of 42 capsules (one bottle contains 14 capsules)
Do not use for more than 14 days in a row unless directed by your doctor. For the 28 count (two 14 day courses) and the 42 count (three 14 day courses), you may repeat a 14 day course every 4 months.
- SPL UNCLASSIFIED SECTION
- Carton and container labels
-
INGREDIENTS AND APPEARANCE
OMEPRAZOLE AND SODIUM BICARBONATE
omeprazole and sodium bicarbonate capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68382-503 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMEPRAZOLE (UNII: KG60484QX9) (OMEPRAZOLE - UNII:KG60484QX9) OMEPRAZOLE 20 mg SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 1100 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) Product Characteristics Color WHITE (OPAQUE WHITE) , WHITE (OPAQUE WHITE) Score no score Shape CAPSULE (CAPSULE) Size 24mm Flavor Imprint Code 503 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68382-503-17 2 in 1 CARTON 07/19/2018 1 14 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68382-503-67 1 in 1 CARTON 07/19/2018 2 14 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:68382-503-04 3 in 1 CARTON 07/19/2018 3 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203345 07/19/2018 Labeler - Zydus Pharmaceuticals USA Inc. (156861945) Registrant - Zydus Worldwide DMCC (557951127) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(68382-503) , MANUFACTURE(68382-503)