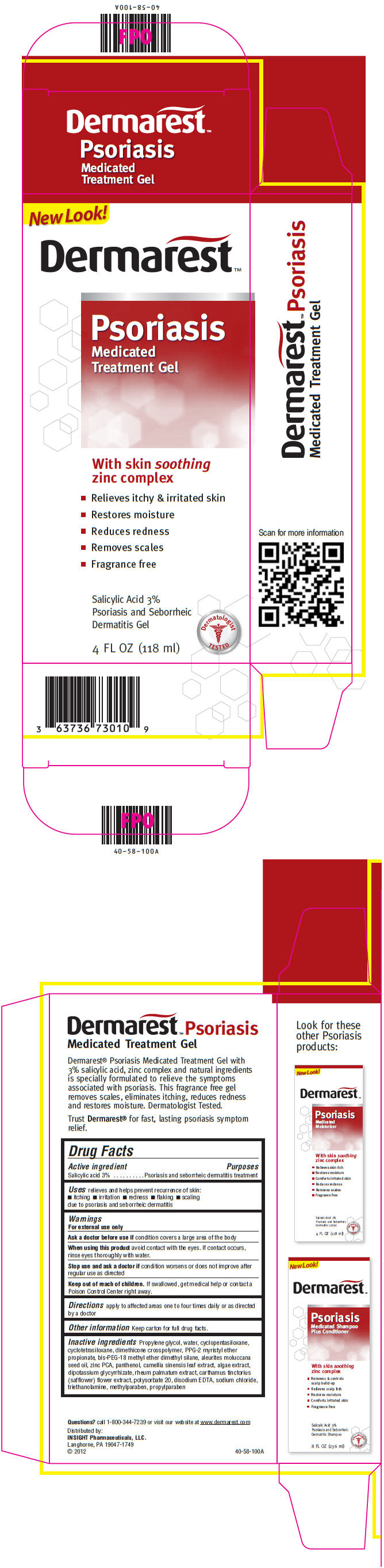
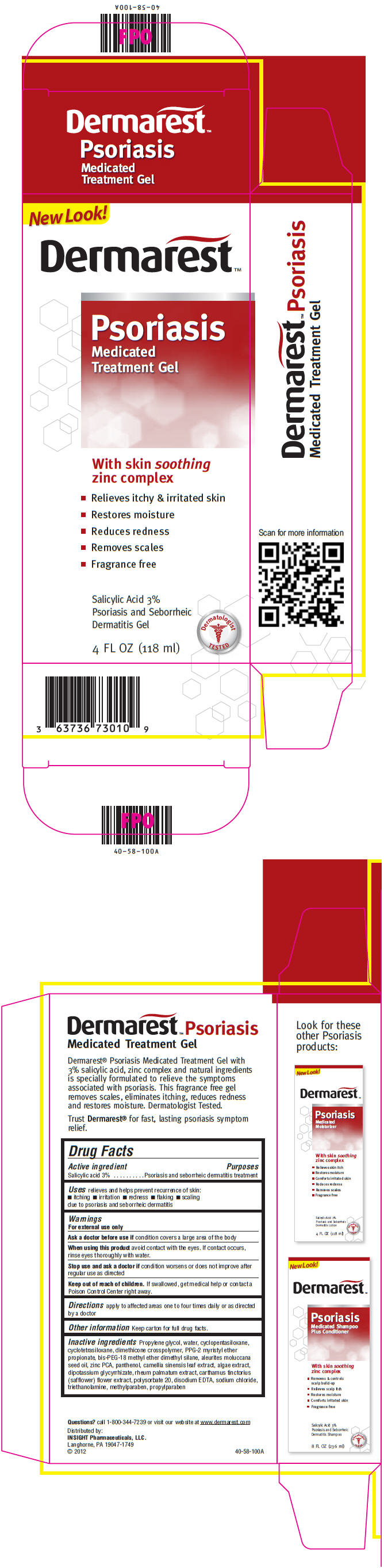
Label: DERMAREST PSORIASIS MEDICATED SKIN TREATMENT- salicylic acid gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 63736-301-24 - Packager: Insight Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 4, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredientSalicylic acid 3%
-
PurposesPsoriasis and seborrheic dermatitis treatment
-
Usesrelieves and helps prevent recurrence of skin: itching - irritation - redness - flaking - scaling - due to psoriasis and seborrheic dermatitis
-
WarningsFor external use only - Ask a doctor before use if condition covers a large area of the body - When using this product avoid contact with the eyes. If contact occurs, rinse eyes thoroughly ...
-
Directionsapply to affected areas one to four times daily or as directed by a doctor
-
Other informationKeep carton for full drug facts.
-
Inactive ingredientsPropylene glycol, water, cyclopentasiloxane, cyclotetrasiloxane, dimethicone crosspolymer, PPG-2 myristyl ether propionate, bis-PEG-18 methyl ether dimethyl silane, aleurites moluccana seed oil ...
-
Questions?call 1-800-344-7239 or visit our website at www.dermarest.com
-
SPL UNCLASSIFIED SECTIONDistributed by: INSIGHT Pharmaceuticals, LLC. Langhorne, PA 19047-1749
-
PRINCIPAL DISPLAY PANEL - 118 ml Bottle CartonNew Look! Dermarest™ Psoriasis - Medicated - Treatment Gel - With skin soothing - zinc complex - Relieves itchy & irritated skin - Restores moisture - Reduces redness - Removes scales - Fragrance ...
-
INGREDIENTS AND APPEARANCEProduct Information