CARDIOVASCULAR EFFECTS
-
Cardiovascular Thrombotic Events
-
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious ...
CARDIOVASCULAR EFFECTS
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as Nabumetone, increases the risk of serious gastrointestinal (GI) events [see WARNINGS].
Status Post Coronary Artery Bypass Graft (CABG) Surgery
Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10 to 14 days following CABG surgery found an increased incidence of myocardial infarction and stroke. NSAIDs are contraindicated in the setting of CABG [see Contraindications].
Post-MI Patients
Observational studies conducted in the Danish National Registry have demonstrated that patients treated with NSAIDs in the post-MI period were at increased risk of reinfarction, CV-related death, and all-cause mortality beginning in the first week of treatment. In this same cohort, the incidence of death in the first year post MI was 20 per 100 person years in NSAID-treated patients compared to 12 per 100 person years in non-NSAID exposed patients. Although the absolute rate of death declined somewhat after the first year post-MI, the increased relative risk of death in NSAID users persisted over at least the next four years of follow-up.
Avoid the use of nabumetone tablets in patients with a recent MI unless the benefits are expected to outweigh the risk of recurrent CV thrombotic events. If nabumetone tablets are used in patients with a recent MI, monitor patients for signs of cardiac ischemia.
Hypertension: NSAIDs, including nabumetone tablets, can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including nabumetone tablets, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Heart Failure and Edema
The Coxib and traditional NSAID Trialists’ Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalizations for heart failure in COX-2 selective-treated patients and nonselective NSAID-treated patients compared to placebo-treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of Nabumetone may blunt the CV effects of several therapeutic agents used to treat these medical conditions [e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers (ARBs)] [see Drug Interactions].
Avoid the use of nabumetone tablets in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If nabumetone tablets are used in patients with severe heart failure, monitor patients for signs of worsening heart failure.
Gastrointestinal Effects, Risk of Ulceration, Bleeding, and Perforation: NSAIDs, including nabumetone tablets, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only 1 in 5 patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3 to 6 months, and in about 2 to 4% of patients treated for 1 year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
In controlled clinical trials involving 1,677 patients treated with nabumetone (1,140 followed for 1 year and 927 for 2 years), the cumulative incidence of peptic ulcers was 0.3% (95% CI; 0%, 0.6%) at 3 to 6 months, 0.5% (95% CI; 0.1%, 0.9%) at 1 year and 0.8% (95% CI; 0.3%, 1.3%) at 2 years. In patients with active peptic ulcer, physicians must weigh the benefits of therapy with nabumetone against possible hazards, institute an appropriate ulcer treatment regimen and monitor the patients’ progress carefully. NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Renal Effects: Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of an NSAID results in a dose-dependent decrease in prostaglandin synthesis and, secondarily, in a reduction of renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease: No information is available from controlled clinical studies regarding the use of nabumetone tablets in patients with advanced renal disease. Therefore, treatment with nabumetone tablets is not recommended in these patients with advanced renal disease. If nabumetone tablets therapy must be initiated, close monitoring of the patient’s renal function is advisable.
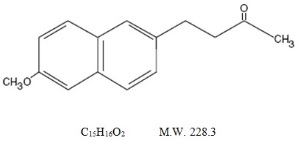
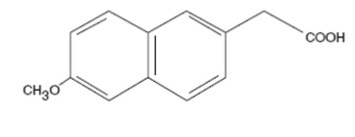
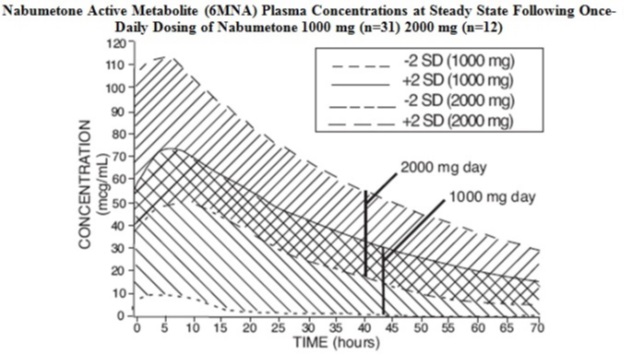
Because nabumetone undergoes extensive hepatic metabolism, no adjustment of the dosage of nabumetone is generally necessary in patients with mild renal insufficiency; however, as with all NSAIDs, patients with impaired renal function should be monitored more closely than patients with normal renal function (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Renal Insufficiency). In subjects with moderate renal impairment (creatinine clearance 30 to 49 mL/min) there is a 50% increase in unbound plasma 6MNA and dose adjustment may be warranted. The oxidized and conjugated metabolites of 6MNA are eliminated primarily by the kidneys.
Anaphylactoid Reactions: As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to nabumetone tablets. Nabumetone tablets should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS, General, Preexisting Asthma). Emergency help should be sought in cases where an anaphylactoid reaction occurs.
Serious Skin Reactions: NSAIDs, including nabumetone, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. NSAIDs can also cause fixed drug eruption (FDE). FDE may present as a more severe variant known as generalized bullous fixed drug eruption (GBFDE), which can be life-threatening. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions and to discontinue the use of NABUMETONE TABLETS at the first appearance of skin rash or any other sign of hypersensitivity.
NABUMETONE TABLET is contraindicated in patients with previous serious skin reactions to NSAIDs (see CONTRAINDICATIONS).
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as nabumetone tablets. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, reaction, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though reaction is not evident. If such signs or symptoms are present, discontinue nabumetone tablets and evaluate the patient immediately.
Fetal Toxicity
Premature Closure of Fetal Ductus Arteriosus: Avoid use of NSAIDs, including nabumetone tablets, in pregnant women at about 30 weeks gestation and later. NSAIDs including nabumetone tablets, increase the risk of premature closure of the fetal ductus arteriosus at approximately this gestational age.
Oligohydramnios/Neonatal Renal Impairment:
Use of NSAIDs, including nabumetone tablets, at about 20 weeks gestation or later in pregnancy may cause fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation.
Oligohydramnios is often, but not always, reversible with treatment discontinuation. Complications of prolonged oligohydramnios may, for example, include limb contractures and delayed lung maturation. In some postmarketing cases of impaired neonatal renal function, invasive procedures such as exchange transfusion or dialysis were required.
If NSAID treatment is necessary between about 20 weeks and 30 weeks gestation, limit nabumetone tablets use to the lowest effective dose and shortest duration possible. Consider ultrasound monitoring of amniotic fluid if nabumetone tablets treatment extends beyond 48 hours. Discontinue nabumetone tablets if oligohydramnios occurs and follow up according to clinical practice [see PRECAUTIONS; Pregnancy].
Close