Label: METHYLPHENIDATE HYDROCHLORIDE solution
- NDC Code(s): 27808-058-01, 27808-059-01
- Packager: Cranbury Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METHYLPHENIDATE HYDROCHLORIDE ORAL SOLUTION safely and - effectively. See full prescribing information for METHYLPHENIDATE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: ABUSE, MISUSE, AND ADDICTION
Methylphenidate hydrochloride oral solution has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including methylphenidate hydrochloride, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing methylphenidate hydrochloride oral solution, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout methylphenidate hydrochloride oral solution treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction [see Warnings and Precautions (5.1) and Drug Abuse and Dependence (9.2)].
Close -
1 INDICATIONS AND USAGE
Methylphenidate hydrochloride oral solution is indicated for the treatment of: • Attention Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years of age and older - ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Pretreatment - Screening - Prior to treating patients with methylphenidate hydrochloride oral solution, assess: for the presence of cardiac disease (i.e., perform a careful history ...
-
3 DOSAGE FORMS AND STRENGTHS
Methylphenidate hydrochloride oral solution is a colorless, grape flavored liquid available in a 500 mL bottle in the following strengths: 5 mg per 5 mL - 10 mg per 5 mL
-
4 CONTRAINDICATIONS
Methylphenidate hydrochloride oral solution is contraindicated in patients: with known hypersensitivity to methylphenidate or other components of methylphenidate hydrochloride oral solution ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Abuse, Misuse, and Addiction - Methylphenidate hydrochloride oral solution has a high potential for abuse and misuse. The use of methylphenidate hydrochloride exposes individuals to the ...
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling: Abuse, misuse, and addiction [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2 ...
-
7 DRUG INTERACTIONS
7.1 Clinically Important Drug Interactions - with Methylphenidate Hydrochloride Oral Solution - Table 1 presents clinically important drug interactions with methylphenidate hydrochloride oral ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including methylphenidate ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled - Substance - Methylphenidate hydrochloride oral solution contains methylphenidate hydrochloride, a Schedule II controlled substance. 9.2 Abuse - Methylphenidate hydrochloride ...
-
10 OVERDOSAGE
Clinical Effects of Overdose - Overdose of CNS stimulants is characterized by the following sympathomimetic effects: Cardiovascular effects including tachyarrhythmias, and hypertension or ...
-
11 DESCRIPTION
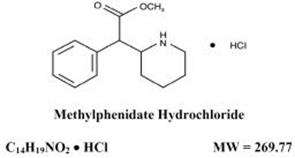
Methylphenidate hydrochloride oral solution is a CNS stimulant available as 5 mg/5 mL and 10 mg/5 mL strengths for oral administration. Chemically, Methylphenidate hydrochloride is (d,l racemic ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Methylphenidate hydrochloride is a central nervous system (CNS) stimulant. The mode of therapeutic action in ADHD is not known. 12.2 ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a lifetime carcinogenicity study carried out in B6C3F1 mice, methylphenidate caused an increase in hepatocellular ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied - Methylphenidate hydrochloride oral solution 5 mg per 5 mL is a colorless, grape flavored liquid. It is supplied in bottles of 500 mL, NDC 27808-058-01. Methylphenidate hydrochloride ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide). Abuse, Misuse, and Addiction - Educate patients and their families about the risks of abuse, misuse, and addiction ...
-
MEDICATION GUIDE
Methylphenidate Hydrochloride Oral Solution CII - (METH il FEN i date) 5 mg/5 mL and 10 mg/5 mL - Rx only - What is the most important information I should know about Methylphenidate ...
-
PRINCIPAL DISPLAY PANELNDC 27808-058-01 - Methylphenidate Hydrochloride Oral Solution CII - 5 mg per 5 mL - PHARMACIST: Dispense the Medication Guide to each patient. Rx only 500 mL
-
PRINCIPAL DISPLAY PANELNDC 27808-059-01 - Methylphenidate Hydrochloride Oral Solution CII - 10 mg per 5 mL - PHARMACIST: Dispense the Medication Guide to each patient. Rx only 500 mL
-
INGREDIENTS AND APPEARANCEProduct Information