Label: NALOXONE HYDROCHLORIDE spray, metered
- NDC Code(s): 0591-2971-54, 0591-2971-99
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NALOXONE HYDROCHLORIDE NASAL SPRAY safely and effectively. See full prescribing information for NALOXONE HYDROCHLORIDE NASAL SPRAY ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENaloxone hydrochloride nasal spray is indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression. Naloxone ...
-
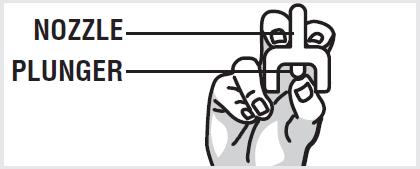
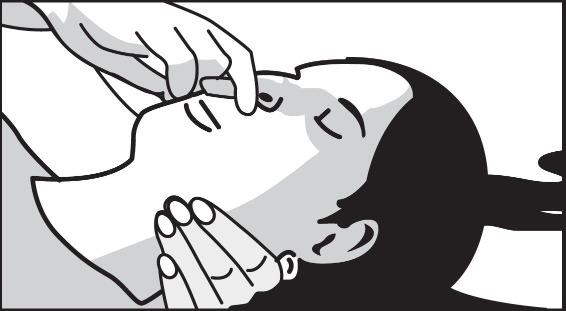
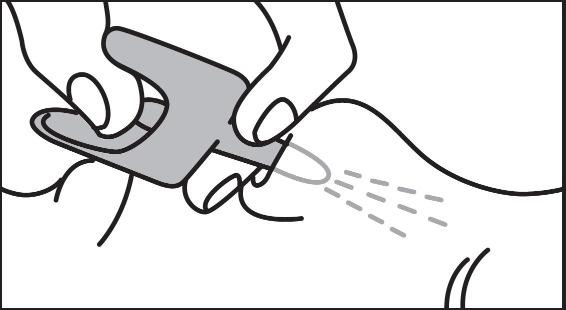
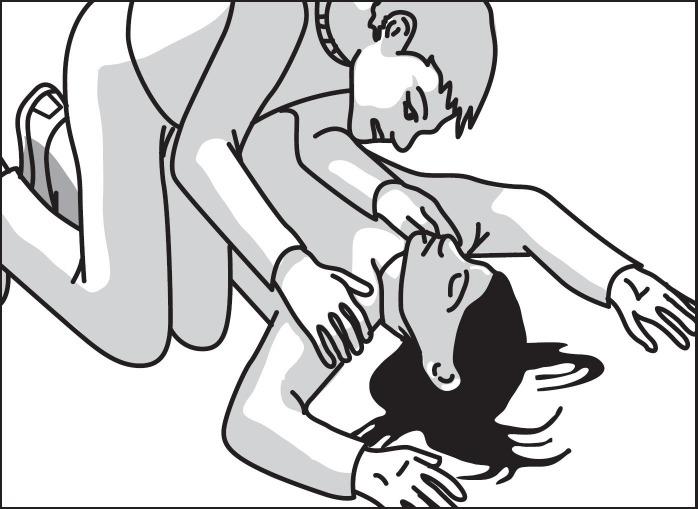
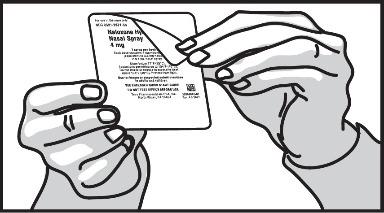
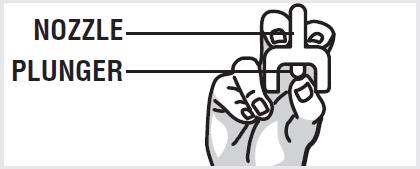
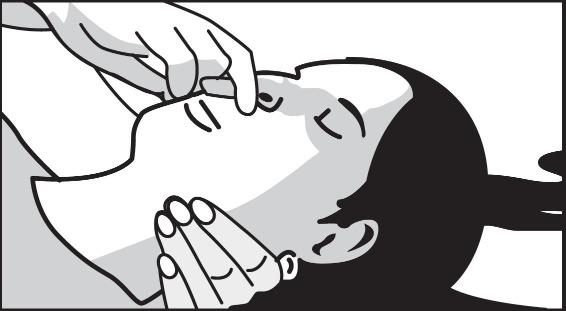
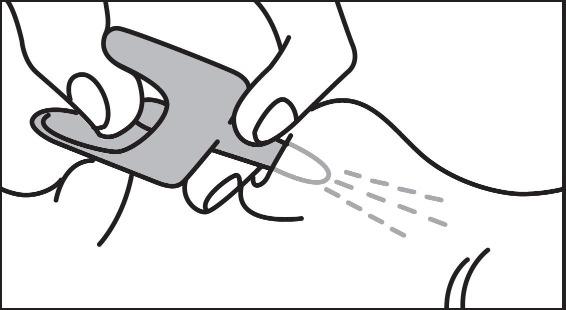
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Naloxone hydrochloride nasal spray is for intranasal use only. No additional device assembly is required. Because treatment of suspected opioid ...
-
3 DOSAGE FORMS AND STRENGTHSNaloxone hydrochloride nasal spray is supplied as a single-dose intranasal spray containing 4 mg of naloxone hydrochloride in 0.1 mL.
-
4 CONTRAINDICATIONSNaloxone hydrochloride nasal spray is contraindicated in patients known to be hypersensitive to naloxone hydrochloride or to any of the other ingredients.
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Recurrent Respiratory and Central Nervous System Depression - The duration of action of most opioids may exceed that of naloxone hydrochloride nasal spray resulting in a return of ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed elsewhere in the labeling: Precipitation of Severe Opioid Withdrawal [see Warnings and Precautions (5.3)] Because clinical studies are ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data on naloxone use in pregnant women are not sufficient to inform a drug-associated risk. However, there are clinical considerations [see ...
-
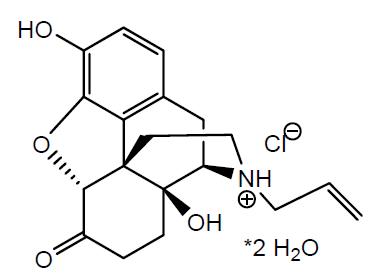
11 DESCRIPTIONNaloxone hydrochloride nasal spray is a pre-filled, single dose intranasal spray. Chemically, naloxone hydrochloride dihydrate is the hydrochloride salt of ...
-
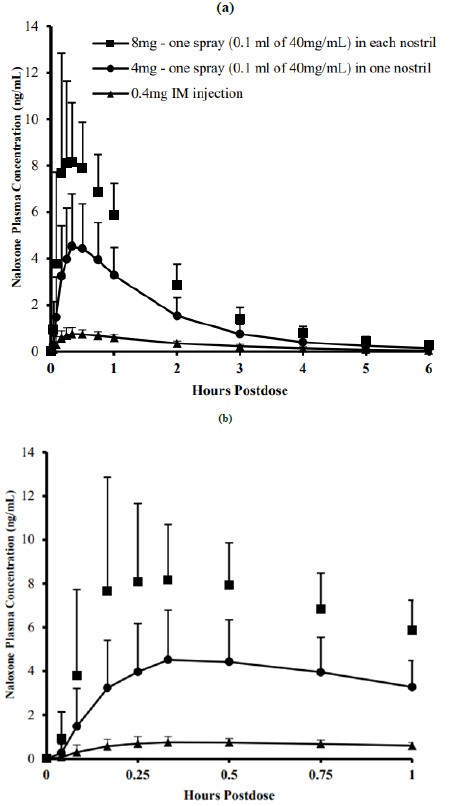
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Naloxone hydrochloride is an opioid antagonist that antagonizes opioid effects by competing for the same receptor sites. Naloxone hydrochloride reverses the effects of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term animal studies to evaluate the carcinogenic potential of naloxone have not been ...
-
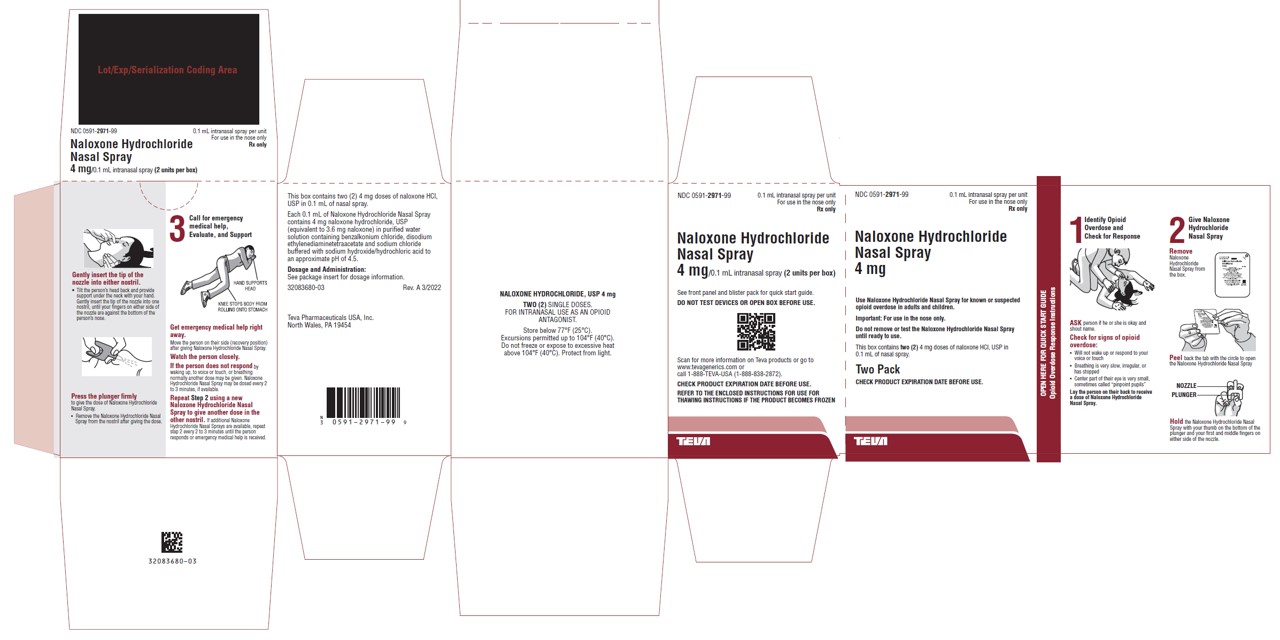
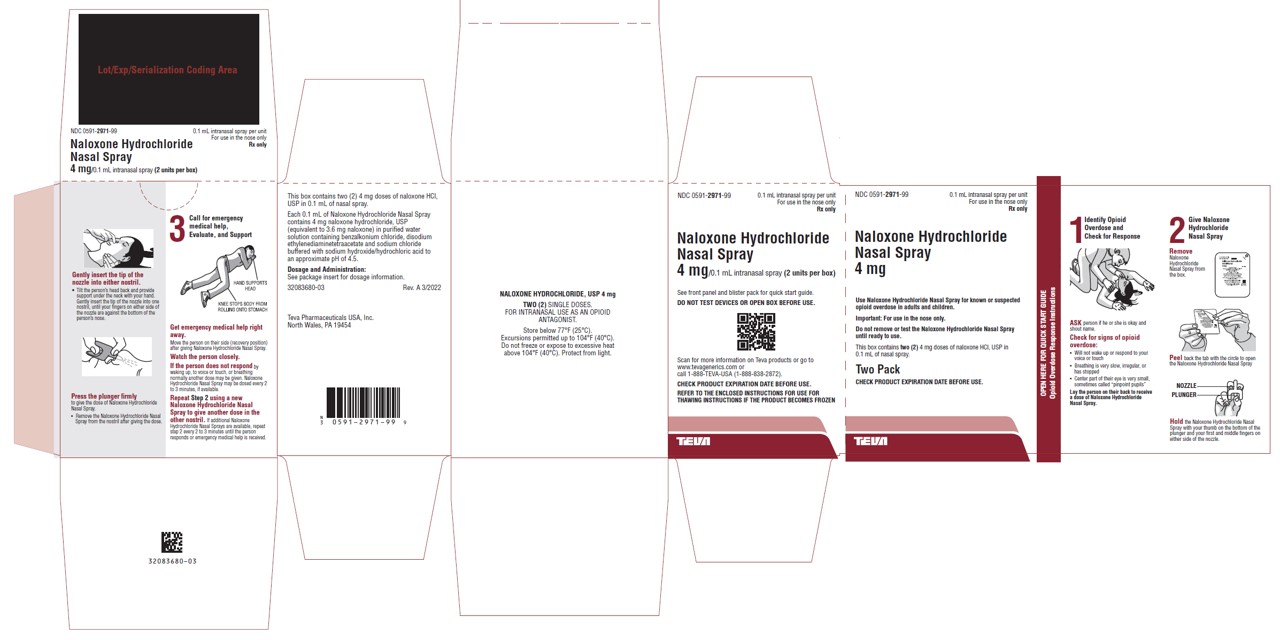
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Naloxone hydrochloride nasal spray 4 mg is supplied as a carton containing two blister packages (NDC 0591-2971-99) each with a single spray device. Naloxone hydrochloride ...
-
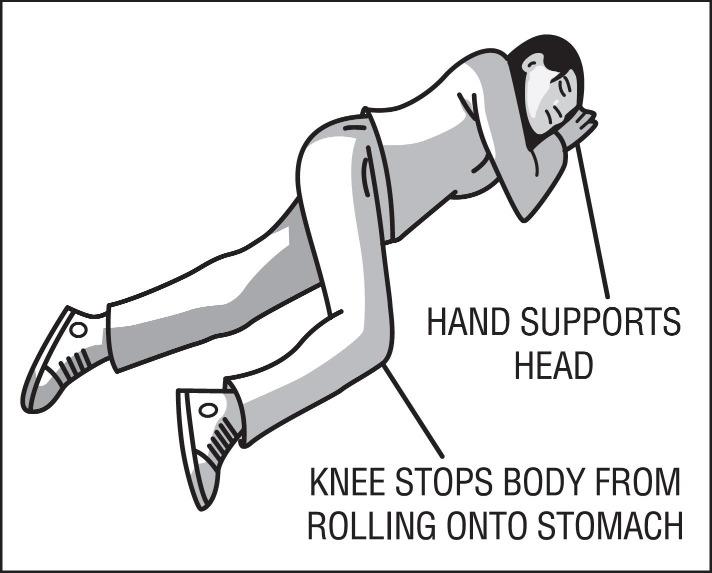
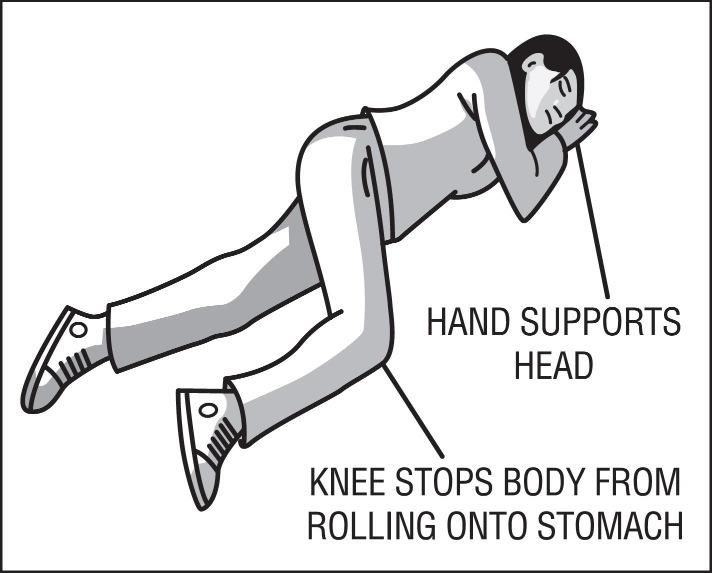
17 PATIENT COUNSELING INFORMATIONAdvise the patient and family members or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Recognition of Opioid Overdose - Inform patients and ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Naloxone Hydrochloride (nal ox’ one hye” droe klor’ ide) Nasal Spray - You and your family members or caregivers should read this Patient Information leaflet ...
-
PRINCIPAL DISPLAY PANELNDC 0591-2971-99 - 0.1 mL intranasal spray per unit - For use in the nose only - Rx only - Naloxone Hydrochloride Nasal Spray - 4 mg - Use Naloxone Hydrochloride Nasal Spray for known or - suspected opioid ...
-
INGREDIENTS AND APPEARANCEProduct Information