Label: ELCYS- cysteine hydrochloride injection, solution
- NDC Code(s): 51754-1007-1, 51754-1007-3
- Packager: Exela Pharma Sciences, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ELCYS safely and effectively. See full prescribing information for ELCYS. Initial U.S. Approval: 1971 - INDICATIONS AND ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE ELCYS is indicated for use as an additive to amino acid solutions to meet the nutritional requirements of newborn infants - requiring total parenteral nutrition (TPN) and of adult and pediatric ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Administration Information - ELCYS is for admixing use only. It is not for direct intravenous infusion. Prior to administration, ELCYS must be diluted - and used as an admixture in ...
-
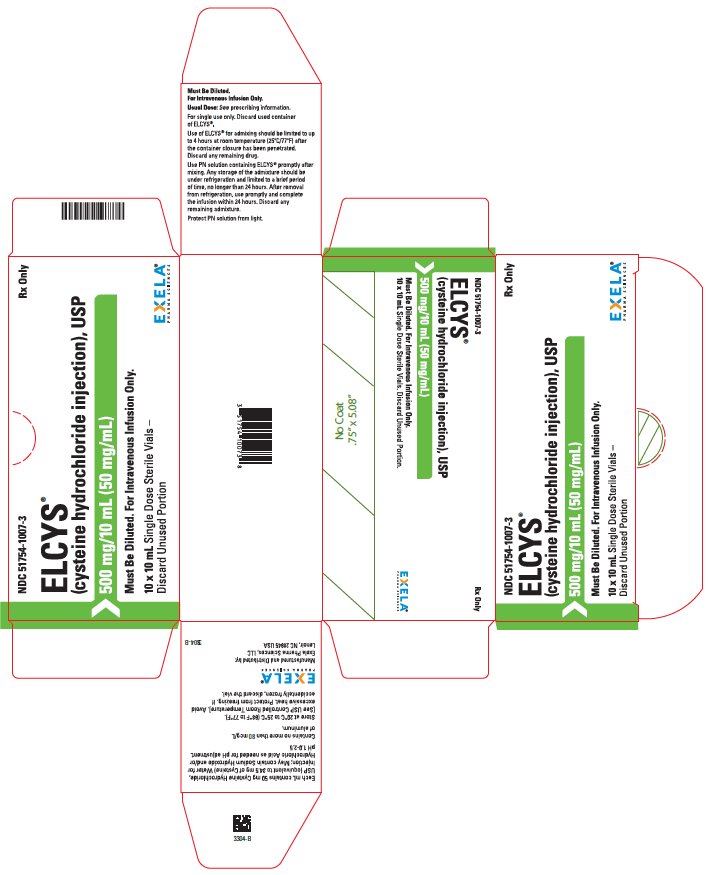
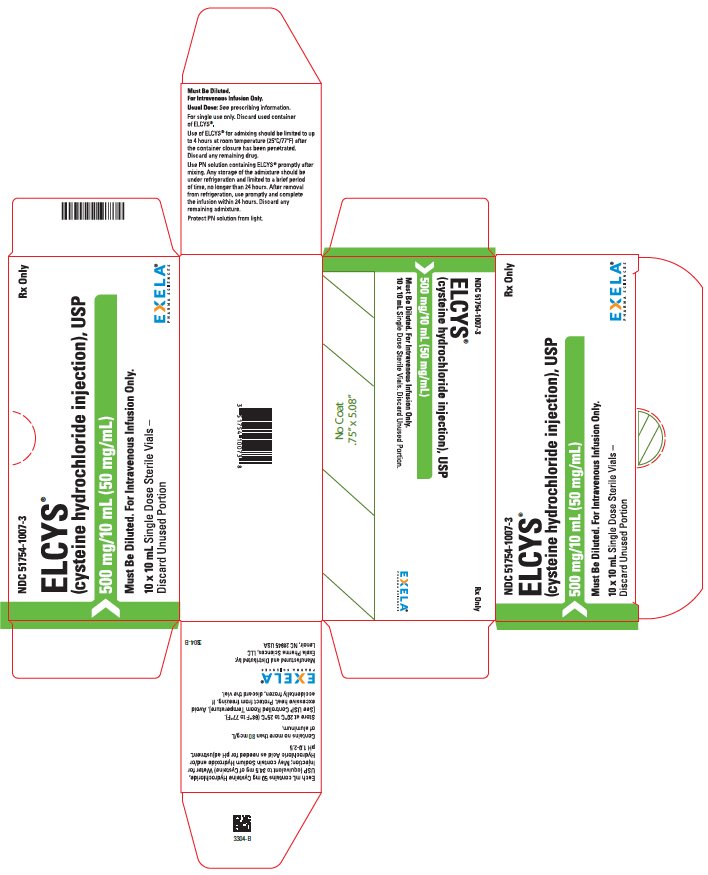
3 DOSAGE FORMS AND STRENGTHS Injection: 500 mg/10 mL (50 mg/mL) cysteine hydrochloride, USP as a clear, colorless, sterile solution in a 10 mL single-dose vial.
-
4 CONTRAINDICATIONS ELCYS is contraindicated in: • Patients with known hypersensitivity to one or more amino acids. • Patients with inborn errors of amino acid metabolism due to risk of severe metabolic or ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Pulmonary Embolism due to Pulmonary Vascular Precipitates - Pulmonary vascular precipitates causing pulmonary vascular emboli and pulmonary distress have been reported in - patients receiving ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed in greater detail in other sections of the prescribing information: • Pulmonary embolism due to pulmonary vascular precipitates [see Warnings ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Appropriate administration of ELCYS is not expected to cause major birth defects, miscarriage or adverse maternal or - fetal outcomes. Animal reproduction studies ...
-
10 OVERDOSAGE In the event of over hydration or solute overload, re-evaluate the patient and institute appropriate corrective measures [see - Warnings and Precautions (5.3, 5.4, 5.5, 5.7, 5.8)].
-
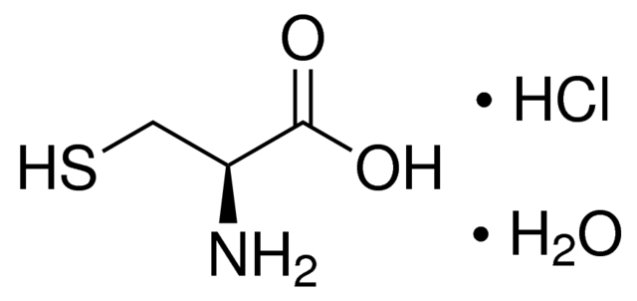
11 DESCRIPTION ELCYS (cysteine hydrochloride injection) is a sterile, nonpyrogenic solution for intravenous use. Each 10 mL of ELCYS - contains 500 mg of cysteine hydrochloride, USP (equivalent to 345 mg of ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Endogenous cysteine is synthesized from methionine by the enzyme, cystathionase, via the trans-sulfuration pathway, and - serves as a precursor substrate for both ...
-
15 REFERENCES 1. Ayers P. et al. A.S.P.E.N. Parenteral Nutrition Handbook, 2nd ed. 2014 pg. 123 and 124.
-
16 HOW SUPPLIED/STORAGE AND HANDLING ELCYS is supplied as follows: 500 mg/10 mL (50 mg/mL) of cysteine hydrochloride, USP is a clear, colorless, sterile and nonpyrogenic solution in 10 - mL single-dose vials (51754-1007-1), packaged as ...
-
17 PATIENT COUNSELING INFORMATION Inform patients, caregivers, or home healthcare providers of the following risks of ELCYS: • Pulmonary embolism due to pulmonary vascular precipitates [see Warnings and Precautions (5.1)] • Vein ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-Vial Label Rx Only NDC 51754-1007-1 - ELCYS - (Cysteine Hydrochloride - Injection), USP - 500 mg/10 mL (50 mg/mL) Must Be Diluted - For Intravenous Use Only After Dilution - 10 mL Single Dose Sterile ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-Carton Label NDC 51754-1007-3 Rx Only - ELCYS - (Cysteine Hydrochloride Injection), USP - 500 mg/10 mL (50 mg/mL) Must Be Diluted. For Intravenous Use Only. 10 x 10 mL Single Dose SterileVials- Discard ...
-
INGREDIENTS AND APPEARANCEProduct Information