Label: PREVICOX- firocoxib tablet, chewable
-
NDC Code(s):
0010-9140-02,
0010-9140-03,
0010-9140-06,
0010-9150-02, view more0010-9150-03, 0010-9150-05
- Packager: Boehringer Ingelheim Animal Health USA Inc
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
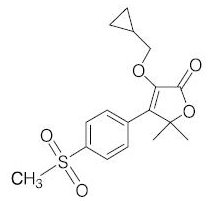
Description: PREVICOX (firocoxib) belongs to the coxib class of non-narcotic, non-steroidal anti-inflammatory drugs. Firocoxib is a white crystalline compound described chemically as 3-(cyclopropylmethoxy)-4-(4-(methylsulfonyl)phenyl)- 5,5-dimethylfuranone. The empirical formula is C17H20O5S, and the molecular weight is 336.4. The structural formula is shown below:
-
PHARMACOKINETICS
Pharmacokinetics: The absolute bioavailability of PREVICOX (firocoxib) is approximately 38% when administered as a 5 mg/kg oral dose to fasted adult dogs. Firocoxib is rapidly cleared from the blood via hepatic metabolism and fecal excretion (CLsystemic = ~0.4 L/hr/kg). Despite a high level of plasma protein binding (96%), firocoxib exhibits a large volume of distribution (Vdλ of total drug = ~4.6 L/kg) and a terminal elimination half life of 7.8 hours (%CV = 30%). The oral drug absorption process is highly variable among subjects. Co-administration of PREVICOX with food delays drug absorption (Tmax from 1 to 5 hours) and decreases peak concentrations (Cmax from 1.3 to 0.9 mcg/mL). However, food does not affect the overall oral bioavailability at the recommended dose.
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Dosage and Administration: Always provide the Client Information Sheet with prescription. Carefully consider the potential benefits and risks of PREVICOX and other treatment options before deciding to use PREVICOX. Use the lowest effective dose for the shortest duration consistent with individual response. The recommended dosage of PREVICOX (firocoxib) for oral administration in dogs is 2.27 mg/lb (5.0 mg/kg) body weight once daily as needed for osteoarthritis and for 3 days as needed for postoperative pain and inflammation associated with soft-tissue and orthopedic surgery. The dogs can be treated with PREVICOX approximately two hours prior to surgery. The tablets are scored and dosage should be calculated in half tablet increments. PREVICOX Chewable Tablets can be administered with or without food.
- CONTRAINDICATIONS
-
WARNINGS
Warnings: Not for use in humans. Keep this and all medications out of the reach of children. Consult a physician in case of accidental ingestion by humans.
For oral use in dogs only. Use of this product at doses above the recommended 2.27 mg/lb (5.0 mg/kg) in puppies less than seven months of age has been associated with serious adverse reactions, including death (see Animal Safety). Due to tablet sizes and scoring, dogs weighing less than 12.5 lb (5.7 kg) cannot be accurately dosed.
All dogs should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory testing to establish hematological and serum baseline data is recommended prior to and periodically during administration of any NSAID.
Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions and Animal Safety) and be given a Client Information Sheet about PREVICOX Chewable Tablets.
For technical assistance or to report suspected adverse events, call 1-888-637-4251.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
-
PRECAUTIONS
Precautions: This product cannot be accurately dosed in dogs less than 12.5 pounds in body weight.
Consider appropriate washout times when switching from one NSAID to another or when switching from corticosteroid use to NSAID use.
As a class, cyclooxygenase inhibitory NSAIDs may be associated with renal, gastrointestinal and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached and monitored. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Since NSAIDS possess the potential to produce gastrointestinal ulceration and/or gastrointestinal perforation, concomitant use of Previcox Chewable Tablets with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided. The concomitant use of protein bound drugs with PREVICOX Chewable Tablets has not been studied in dogs. Commonly used protein-bound drugs include cardiac, anticonvulsant, and behavioral medications. The influence of concomitant drugs that may inhibit the metabolism of PREVICOX Chewable Tablets has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy.
If additional pain medication is needed after the daily dose of PREVICOX, a non-NSAID class of analgesic may be necessary.
Appropriate monitoring procedures should be employed during all surgical procedures. Anesthetic drugs may affect renal perfusion, approach concomitant use of anesthetics and NSAIDs cautiously. The use of parenteral fluids during surgery should be considered to decrease potential renal complications when using NSAIDs perioperatively.
The safe use of PREVICOX Chewable Tablets in pregnant, lactating or breeding dogs has not been evaluated.
-
ADVERSE REACTIONS
Adverse Reactions:
Osteoarthritis: In controlled field studies, 128 dogs (ages 11 months to 15 years) were evaluated for safety when given PREVICOX Chewable Tablets at a dose of 2.27 mg/lb (5.0 mg/kg) orally once daily for 30 days. The following adverse reactions were observed. Dogs may have experienced more than one of the observed adverse reactions during the study.
Adverse Reactions Seen in U.S. Field Studies Adverse Reactions Previcox
n=128Active Control
n=121Vomiting
5
8
Diarrhea
1
10
Decreased Appetite or Anorexia
3
3
Lethargy
1
3
Pain
2
1
Somnolence
1
1
Hyperactivity
1
0
PREVICOX (firocoxib) Chewable Tablets were safely used during field studies concomitantly with other therapies, including vaccines, anthelmintics, and antibiotics.
Soft-tissue Surgery: In controlled field studies evaluating soft-tissue postoperative pain and inflammation, 258 dogs (ages 10.5 weeks to 16 years) were evaluated for safety when given PREVICOX Chewable Tablets at a dose of 2.27 mg/lb (5.0 mg/kg) orally approximately 2 hours prior to surgery and once daily thereafter for up to two days. The following adverse reactions were observed. Dogs may have experienced more than one of the observed reactions during the study.
Orthopedic Surgery: In a controlled field study evaluating orthopedic postoperative pain and inflammation, 226 dogs of various breeds, ranging in age from 1 to 11.9 years in the PREVICOX-treated groups and 0.7 to 17 years in the control group were evaluated for safety. Of the 226 dogs, 118 were given PREVICOX Chewable Tablets at a dose of 2.27 mg/lb (5.0 mg/kg) orally approximately 2 hours prior to surgery and once daily thereafter for a total of three days. The following adverse reactions were observed. Dogs may have experienced more than one of the observed reactions during the study.
Adverse Reactions Seen in the Orthopedic Surgery Postoperative Pain Field Study Adverse Reactions Firocoxib Group
n=118Control Group*
n=108A case may be represented in more than one category. Vomiting
1
0
Diarrhea
2†
1
Bruising at Surgery Site
2
3
Inappetence/Decreased Appetite
1
2
Pyrexia
0
1
Incision Swelling, Redness
9
5
Oozing Incision
2
0
Post-Approval Experience (Rev. 2009): The following adverse reactions are based on post-approval adverse drug event reporting. The categories are listed in decreasing order of frequency by body system:
Gastrointestinal: vomiting, anorexia, diarrhea, melena, gastrointestinal perforation, hematemesis, hematachezia, weight loss, gastrointestinal ulceration, peritonitis, abdominal pain, hypersalivation, nausea
Urinary: elevated BUN, elevated creatinine, polydypsia, polyuria, hematuria, urinary incontinence, proteinuria, kidney failure, azotemia, urinary tract infection
Neurological/Behavioral/Special Sense: depression/lethargy, ataxia, seizures, nervousness, confusion, weakness, hyperactivity, tremor, paresis, head tilt, nystagmus, mydriasis, aggression, uveitis.
Hepatic: elevated ALP, elevated ALT, elevated bilirubin, decreased albumin, elevated AST, icterus, decreased or increased total protein and globulin, pancreatitis, ascites, liver failure, decreased BUN
Hematological: anemia, neutrophilia, thrombocytopenia, neutropenia
Cardiovascular/Respiratory: tachypnea, dyspnea, tachycardia
Dermatologic/Immunologic: pruritis, fever, alopecia, moist dermatitis, autoimmune hemolytic anemia, facial/muzzle edema, urticaria
In some cases, death has been reported as an outcome of the adverse events listed above.
For technical assistance or to report suspected adverse events, call 1-888-637-4251. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
-
INFORMATION FOR OWNERS/CAREGIVERS
Information For Dog Owners: PREVICOX, like other drugs of its class, is not free from adverse reactions. Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include vomiting, diarrhea, decreased appetite, dark or tarry stools, increased water consumption, increased urination, pale gums due to anemia, yellowing of gums, skin or white of the eye due to jaundice, lethargy, incoordination, seizure, or behavioral changes. Serious adverse reactions associated with this drug class can occur without warning and in rare situations result in death (see Adverse Reactions). Owners should be advised to discontinue PREVICOX therapy and contact their veterinarian immediately if signs of intolerance are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is withdrawn, and veterinary care, if appropriate, is initiated. Owners should be advised of the importance of periodic follow up for all dogs during administration of any NSAID.
-
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
Clinical Pharmacology: Mode of action: PREVICOX (firocoxib) is a cyclooxygenase-inhibiting (coxib) class, non-narcotic, non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory and analgesic properties. There are two main cyclooxygenase enzymes, COX-1 and COX-2, and a newly discovered third enzyme, COX-3, which has yet to be fully characterized.1 Cyclooxygenase-1 (COX-1) is the enzyme responsible for facilitating constitutive physiologic processes, e.g., platelet aggregation, gastric mucosal protection, and renal perfusion.2 It also is constitutively expressed in the brain, spinal cord, and reproductive tract.3 Cyclooxygenase-2 (COX-2) is responsible for the synthesis of inflammatory mediators, but it is also constitutively expressed in the brain, spinal cord and kidneys.4, 5, 6 Cyclooxygenase-3 (COX-3) is also constitutively expressed in the canine and human brain and also the human heart.7 Results from in vitro studies showed firocoxib to be highly selective for the COX-2 enzyme when canine blood was exposed to drug concentrations comparable to those observed following a once daily 5 mg/kg oral dose in dogs.8 However, the clinical significance of these findings has not been established.
-
SPL UNCLASSIFIED SECTION
Effectiveness: Two hundred and forty-nine dogs of various breeds, ranging in age from 11 months to 20 years, and weighing 13 to 175 lbs, were randomly administered PREVICOX or an active control drug in two field studies. Dogs were assessed for lameness, pain on manipulation, range of motion, joint swelling, and overall improvement in a non-inferiority evaluation of PREVICOX compared with the active control. At the study's end, 87% of the owners rated PREVICOX-treated dogs as improved. Eighty-eight percent of dogs treated with PREVICOX were also judged improved by the veterinarians. Dogs treated with PREVICOX showed a level of improvement in veterinarian-assessed lameness, pain on palpation, range of motion, and owner-assessed improvement that was comparable to the active control. The level of improvement in PREVICOX-treated dogs in limb weight bearing on the force plate gait analysis assessment was comparable to the active control.
In a separate field study, two hundred fifty-eight client-owned dogs of various breeds, ranging in age from 10.5 weeks to 16 years and weighing from 7 to 168 lbs, were randomly administered PREVICOX or a control (sham-dosed-pilled) for the control of postoperative pain and inflammation associated with soft-tissue surgical procedures such as abdominal surgery (e.g. ovariohysterectomy, abdominal cryptorchidectomy, splenectomy, cystotomy) or major external surgeries (e.g. mastectomy, skin tumor removal ≥8 cm). The study demonstrated that PREVICOX-treated dogs had significantly lower need for rescue medication than the control (sham-dosed-pilled) in controlling postoperative pain and inflammation associated with soft-surgery.
A multi-center field study with 226 client-owned dogs of various breeds, and ranging in age from 1 to 11.9 years in the PREVICOX-treated groups and 0.7 to 17 years in the control group was conducted. Dogs were randomly assigned to either the PREVICOX or the control (sham-dosed-pilled) group for the control of postoperative pain and inflammation associated with orthopedic surgery. Surgery to repair a ruptured cruciate ligament included the following stabilization procedures: fabellar suture and/or imbrication, fibular head transposition, tibial plateau leveling osteotomy (TPLO), and 'over the top' technique. The study (n = 220 for effectiveness) demonstrated that PREVICOX-treated dogs had significantly lower need for rescue medication than the control (sham-dosed-pilled) in controlling postoperative pain and inflammation associated with orthopedic surgery.
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
Animal Safety: In a target animal safety study, firocoxib was administered orally to healthy adult Beagle dogs (eight dogs per group) at 5, 15, and 25 mg/kg (1, 3, and 5 times the recommended total daily dose) for 180 days. At the indicated dose of 5 mg/kg, there were no treatment related adverse events. Decreased appetite, vomiting, and diarrhea were seen in dogs in all dose groups, including unmedicated controls, although vomiting and diarrhea were seen more often in dogs in the 5X dose group. One dog in the 3X dose group was diagnosed with juvenile polyarteritis of unknown etiology after exhibiting recurrent episodes of vomiting and diarrhea, lethargy, pain, anorexia, ataxia, proprioceptive deficits, decreased albumin levels, decreased and then elevated platelet counts, increased bleeding times, and elevated liver enzymes. On histopathologic examination, a mild ileal ulcer was found in one 5X dog. This dog also had a decreased serum albumin which returned to normal by study completion. One control and three 5X dogs had focal areas of inflammation in the pylorus or small intestine. Vacuolization without inflammatory cell infiltrates was noted in the thalamic region of the brain in three control, one 3X, and three 5X dogs. Mean ALP was within the normal range for all groups but was greater in the 3X and 5X dose groups than in the control group. Transient decreases in serum albumin were seen in multiple animals in the 3X and 5X dose groups, and in one control animal.
In a separate safety study, firocoxib was administered orally to healthy juvenile (10-13 weeks of age) Beagle dogs at 5, 15, and 25 mg/kg (1, 3, and 5 times the recommended total daily dose) for 180 days. At the indicated (1X) dose of 5 mg/kg, on histopathologic examination, three out of six dogs had minimal periportal hepatic fatty change. On histopathologic examination, one control, one 1X, and two 5X dogs had diffuse slight hepatic fatty change. These animals showed no clinical signs and had no liver enzyme elevations. In the 3X dose group, one dog was euthanized because of poor clinical condition (Day 63). This dog also had a mildly decreased serum albumin. At study completion, out of five surviving and clinically normal 3X dogs, three had minimal periportal hepatic fatty change. Of twelve dogs in the 5X dose group, one died (Day 82) and three moribund dogs were euthanized (Days 38, 78, and 79) because of anorexia, poor weight gain, depression, and in one dog, vomiting. One of the euthanized dogs had ingested a rope toy. Two of these 5X dogs had mildly elevated liver enzymes. At necropsy all five of the dogs that died or were euthanized had moderate periportal or severe panzonal hepatic fatty change; two had duodenal ulceration; and two had pancreatic edema. Of two other clinically normal 5X dogs (out of four euthanized as comparators to the clinically affected dogs), one had slight and one had moderate periportal hepatic fatty change. Drug treatment was discontinued for four dogs in the 5X group. These dogs survived the remaining 14 weeks of the study. On average, the dogs in the 3X and 5X dose groups did not gain as much weight as control dogs. Rate of weight gain was measured (instead of weight loss) because these were young growing dogs. Thalamic vacuolation was seen in three of six dogs in the 3X dose group, five of twelve dogs in the 5X dose group, and to a lesser degree in two unmedicated controls. Diarrhea was seen in all dose groups, including unmedicated controls.
In a separate dose tolerance safety study involving a total of six dogs (two control dogs and four treated dogs), firocoxib was administered to four healthy adult Beagle dogs at 50 mg/kg (ten times the recommended daily dose) for twenty-two days. All dogs survived to the end of the study. Three of the four treated dogs developed small intestinal erosion or ulceration. Treated dogs that developed small intestinal erosion or ulceration had a higher incidence of vomiting, diarrhea, and decreased food consumption than control dogs. One of these dogs had severe duodenal ulceration, with hepatic fatty change and associated vomiting, diarrhea, anorexia, weight loss, ketonuria, and mild elevations in AST and ALT. All four treated dogs exhibited progressively decreasing serum albumin that, with the exception of one dog that developed hypoalbuminemia, remained within normal range. Mild weight loss also occurred in the treated group. One of the two control dogs and three of the four treated dogs exhibited transient increases in ALP that remained within normal range.
- STORAGE AND HANDLING
- HOW SUPPLIED
-
REFERENCES
- 1.
- Willoughby DA, Moore AR and Colville-Nash PR. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet 2000;355:646-648.
- 2.
- Smith, et al., Pharmacological Analysis of Cyclo-oxygenase-1 in Inflammation. Proc. Natl. Acad. Sci. USA, Pharmacology 1998;95:13313-13318.
- 3.
- Jones CJ and Budsberg SC. Physiologic characteristics and clinical importance of the cyclooxygenase isoforms in dogs and cats. JAVMA 2000;217(5):721-729.
- 4.
- Zhang, et al., Inhibition of Cyclo-oxygenase-2 Rapidly Reverses Inflammatory Hyperalgesia and Prostaglandin E2 Production. JPET 1997;283:1069-1075.
- 5.
- Jones and Budsberg, pp. 721-729.
- 6.
- Zhang, et al., pp. 1069-1075.
- 7.
- Chandrasekharan NV, Dai H, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure and expression. Proc. Natl. Acad. Sci. USA, 2002;99(21):13926-13931.
- 8.
- Data on file.
-
SPL UNCLASSIFIED SECTION
Made in France
Marketed by: Boehringer Ingelheim Animal Health USA Inc., Duluth, GA 30096.
1-888-637-4251
Approved by FDA under NADA # 141-230
©2019 Boehringer Ingelheim Animal Health USA Inc. All Rights Reserved.
®PREVICOX is a registered trademark of Boehringer Ingelheim Animal Health USA Inc.Rev. 12-2018
BOEHRINGER INGELHEIM
-
Information for Dog Owners about PREVICOX® (firocoxib) Chewable Tablets
PREVICOX Chewable Tablets are used for the control of pain and inflammation due to osteoarthritis or associated with soft-tissue and orthopedic surgery in your dog.
This summary contains important information about PREVICOX. You should read this information before you start giving your dog PREVICOX tablets and review it each time your prescription is refilled. This sheet is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or you want to know more about PREVICOX.
What is PREVICOX?
PREVICOX is a veterinary prescription non-steroidal anti-inflammatory drug (NSAID) used to control pain and inflammation due to osteoarthritis, or associated with soft-tissue and orthopedic surgery in dogs.
Osteoarthritis is a painful condition caused by "wear and tear" of cartilage and other parts of the joints that may result in the following changes or signs in your dog:
- •
- Limping or lameness.
- •
- Decreased activity or exercise (reluctance to stand, climb stairs, jump or run, or difficulty in performing these activities).
- •
- Stiffness or decreased movement of joints.
PREVICOX is indicated for the control of postoperative pain and inflammation following soft-tissue and orthopedic surgeries (e.g. spays, cruciate ligament repair). Your veterinarian may administer PREVICOX before the procedure and recommend that the dog be treated for a few days after going home.
What kind of results can I expect when my dog is on PREVICOX for osteoarthritis?
While PREVICOX is not a cure for osteoarthritis, it can control the pain and inflammation and improve your dog's mobility.
- •
- Response varies from dog to dog, but improvement can be quite dramatic.
- •
- In most dogs, improvement can be seen within days.
- •
- If PREVICOX is discontinued or not given as directed, your dog's pain and inflammation may return.
What kind of results can I expect when my dog is on PREVICOX for the control of pain and inflammation following soft-tissue and orthopedic surgery?
- •
- PREVICOX Chewable Tablets allow your dog to recover more comfortably by controlling pain and inflammation following soft-tissue and orthopedic surgery.
- •
- Control of pain and inflammation may vary from dog to dog.
- •
- If PREVICOX Chewable Tablets are not given according to your veterinarian's directions, your dog's pain may return.
- •
- Consult your veterinarian if your dog appears to be uncomfortable.
Which dogs should not take PREVICOX?
Your dog should not be given PREVICOX if he/she:
- •
- Has an allergic reaction to firocoxib, the active ingredient in PREVICOX.
- •
- Has had an allergic reaction (such as hives, facial swelling, or red or itchy skin) to aspirin or other NSAIDs.
- •
- Is presently taking aspirin, other NSAIDs, or corticosteroids.
- •
- Is under 12.5 pounds in body weight.
- •
- Has pre-existing kidney or liver disease.
- •
- Has decreased appetite, vomiting or diarrhea.
PREVICOX should only be given to dogs.
People should not take PREVICOX. Keep PREVICOX and all medications out of the reach of children. Call your physician immediately if you accidentally take PREVICOX.
What to tell/ask your veterinarian before giving PREVICOX.
Talk to your veterinarian about:
- •
- The signs of osteoarthritis you have observed in your dog, such as limping or stiffness.
- •
- The importance of weight control in the management of osteoarthritis.
- •
- What tests might be done before PREVICOX is prescribed.
- •
- How often your dog may need to be examined by your veterinarian.
- •
- The risks and benefits of using PREVICOX. Serious adverse reactions, including death, have been associated with PREVICOX administration at doses above the recommended dose in puppies less than seven months of age.
Tell your veterinarian if your dog is currently experiencing or has ever had the following medical problems:
- •
- Any side effects from taking PREVICOX or other NSAIDs, such as aspirin.
- •
- Any digestive upset (vomiting and/or diarrhea).
- •
- Any kidney disease.
- •
- Any liver disease.
Tell your veterinarian about:
- •
- Any other medical problems or allergies that your dog has now, or has had in the past.
- •
- All medicines that you are giving or plan to give to your dog, including those you can get without a prescription and any dietary supplements.
Tell your veterinarian if your dog:
- •
- Is under 7 months of age.
- •
- Is pregnant, nursing or if you plan to breed your dog.
How to give PREVICOX to your dog.
PREVICOX should be given according to your veterinarian's instructions. Do not change the way you give PREVICOX to your dog without first speaking with your veterinarian. Your veterinarian will tell you what amount of PREVICOX is right for your dog and for how long it should be given. Most dogs will take PREVICOX Chewable Tablets from your hand, or you can place the tablet in your dog's mouth. PREVICOX may be given with or without food.
What are the possible side effects that may occur in my dog during PREVICOX therapy?
PREVICOX, like other NSAIDS, may cause some side effects. Serious side effects associated with NSAID therapy in dogs can occur with or without warning, and, in rare situations, result in death. The most common side effects associated with PREVICOX therapy involve the digestive tract (vomiting and decreased food consumption). Liver and kidney problems have also been reported with NSAIDs. Look for the following side effects that may indicate your dog is having a problem with PREVICOX:
- •
- Decrease or increase in appetite.
- •
- Vomiting.
- •
- Change in bowel movements (such as diarrhea, or black, tarry or bloody stools).
- •
- Change in behavior (such as decreased or increased activity level, incoordination, seizure, or aggression).
- •
- Yellowing of gums, skin, or whites of the eyes (jaundice).
- •
- Change in drinking habits (frequency or amount consumed).
- •
- Change in urination habits (frequency, color, or smell).
- •
- Change in skin (redness, scabs, or scratching).
- •
- Unexpected weight loss.
It is important to stop the medication and contact your veterinarian immediately if you think your dog has a medical problem or side effect while taking PREVICOX tablets. If you have additional questions about possible side effects, talk with your veterinarian or call 1-888-637-4251.
Can PREVICOX be given with other medications?
PREVICOX should not be given with other NSAIDs (for example, aspirin, carprofen, etodolac, deracoxib, meloxicam, or tepoxalin) or corticosteroids (for example, prednisone, cortisone, dexamethasone, or triamcinolone).
Tell your veterinarian about all medications that you have given your dog in the past, and any medications you are planning to give with PREVICOX tablets. This should include other medicines that you can get without a prescription or any dietary supplements. Your veterinarian may want to check that all of your dog's medicines can be given together.
What do I do in case my dog eats more than the prescribed amount of PREVICOX?
Consult your veterinarian immediately if your dog eats more than the prescribed amount of PREVICOX.
What else should I know about PREVICOX?
- •
- This sheet provides a summary of information about PREVICOX tablets. If you have any questions or concerns about PREVICOX, osteoarthritis pain, or postoperative pain following soft-tissue and orthopedic surgery, talk with your veterinarian.
- •
- As with all prescribed medicines, PREVICOX tablets should only be given to the dog for which they were prescribed. They should be given to your dog only for the condition for which they were prescribed, at the prescribed dose.
- •
- It is important to periodically discuss your dog's response to PREVICOX tablets. Your veterinarian will determine if your dog is responding as expected and if your dog should continue receiving PREVICOX tablets.
For technical assistance or to report suspected adverse reactions, call 1-888-637-4251.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
Approved by FDA under NADA # 141-230
©2019 Boehringer Ingelheim Animal Health USA Inc. All Rights Reserved.
®PREVICOX is a registered trademark of Boehringer Ingelheim Animal Health USA Inc.Rev. 12-2018
BOEHRINGER INGELHEIM
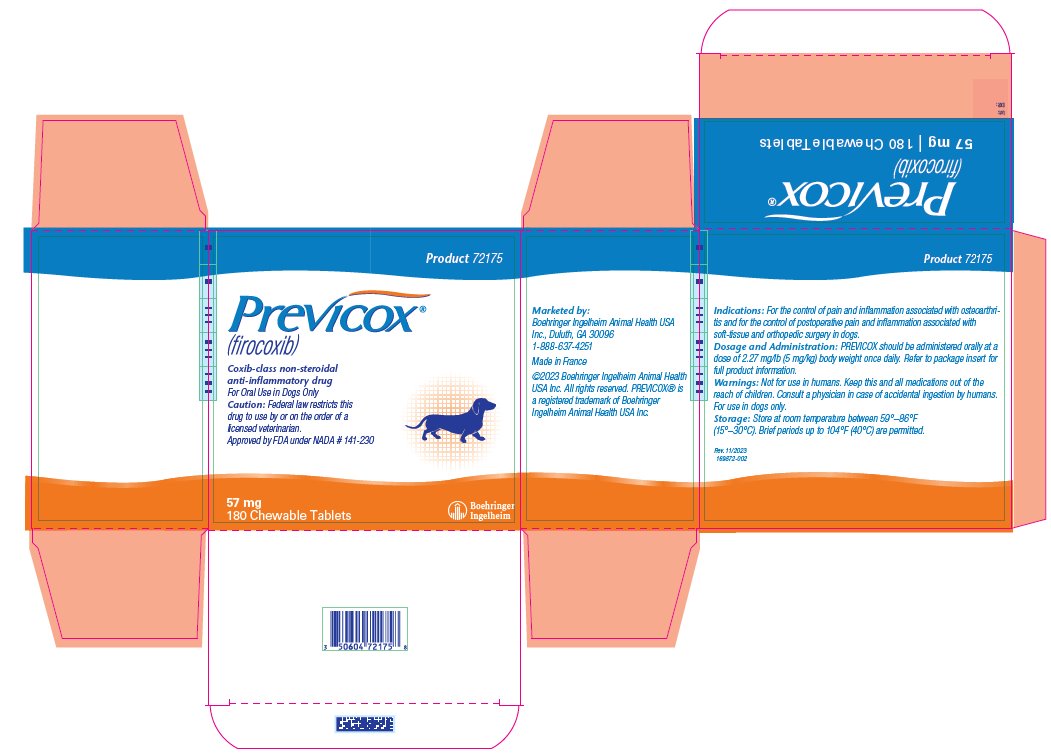
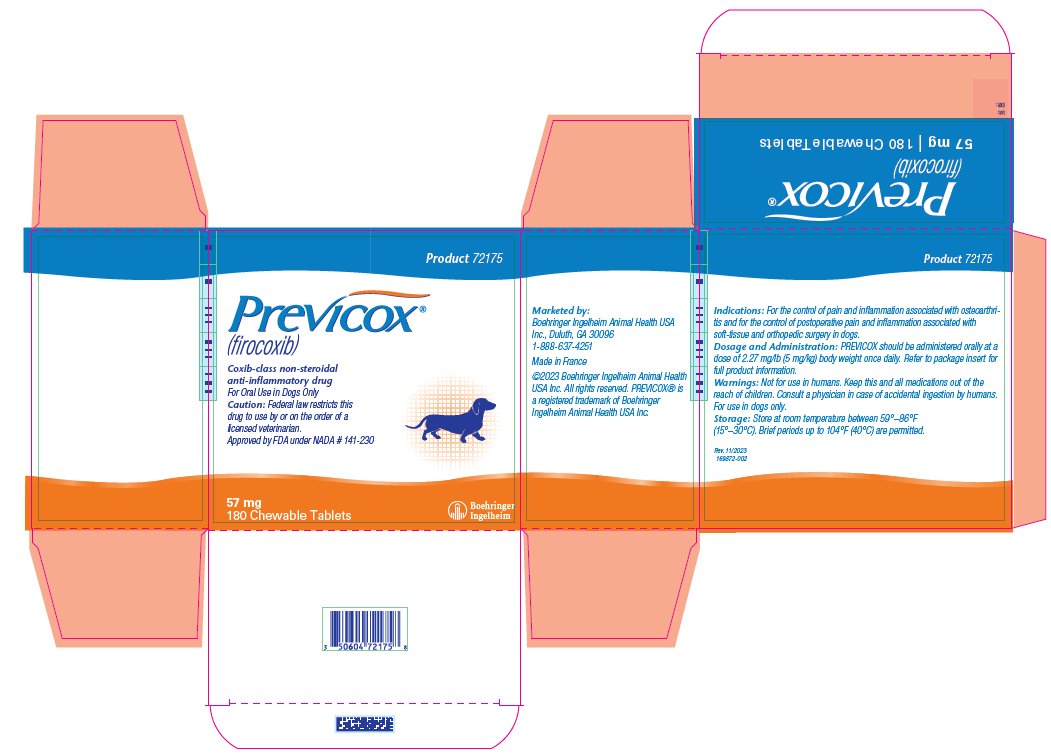
- PRINCIPAL DISPLAY PANEL - 57 mg Tablet Bottle Carton
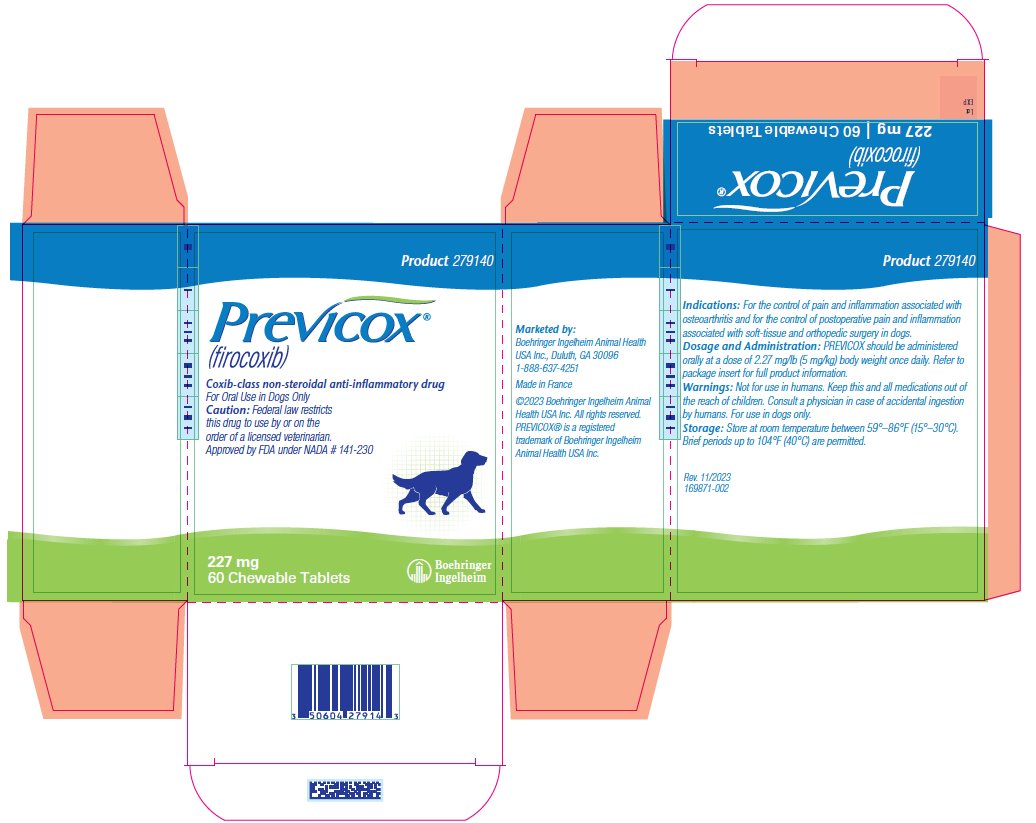
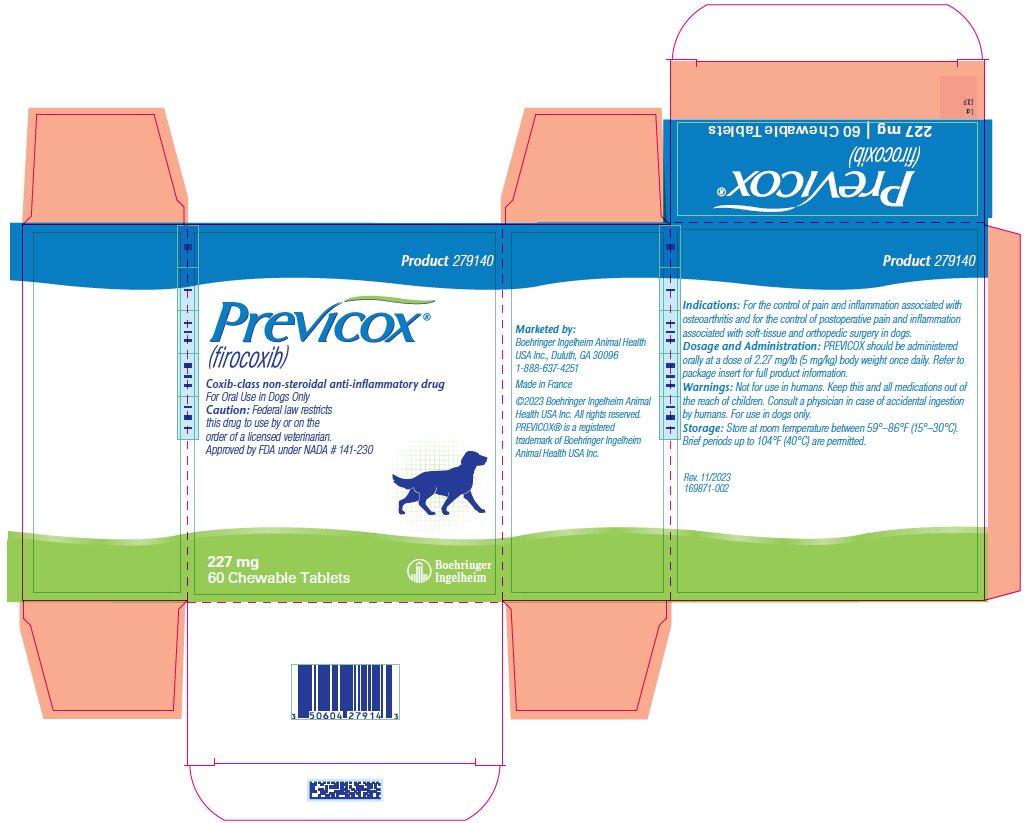
- PRINCIPAL DISPLAY PANEL - 227 mg Tablet Bottle Carton
-
INGREDIENTS AND APPEARANCE
PREVICOX
firocoxib tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-9150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIROCOXIB (UNII: Y6V2W4S4WT) (FIROCOXIB - UNII:Y6V2W4S4WT) FIROCOXIB 57 mg Product Characteristics Color BROWN (Tan/Beige) Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code M;57 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-9150-02 3 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC:0010-9150-03 1 in 1 CARTON 2 60 in 1 BOTTLE, PLASTIC 3 NDC:0010-9150-05 1 in 1 CARTON 3 180 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141230 07/20/2020 PREVICOX
firocoxib tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-9140 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIROCOXIB (UNII: Y6V2W4S4WT) (FIROCOXIB - UNII:Y6V2W4S4WT) FIROCOXIB 227 mg Product Characteristics Color BROWN (Tan/Beige) Score 2 pieces Shape ROUND Size 15mm Flavor Imprint Code M;227 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-9140-02 3 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC:0010-9140-03 1 in 1 CARTON 2 60 in 1 BOTTLE, PLASTIC 3 NDC:0010-9140-06 1 in 1 CARTON 3 180 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141230 07/15/2020 Labeler - Boehringer Ingelheim Animal Health USA Inc (007134091)