Label: GOLDEN MEDICATED- medicated lotion soap solution
- NDC Code(s): 50865-060-09, 50865-060-27
- Packager: Kutol Products Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOLDEN MEDICATED
medicated lotion soap solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50865-060 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) COCONUT ACID (UNII: 40U37V505D) SODIUM LAURYL SULFATE (UNII: 368GB5141J) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) SODIUM SULFATE (UNII: 0YPR65R21J) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) EDETATE SODIUM (UNII: MP1J8420LU) TALL OIL ACID (UNII: H9HR63474M) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) COCO DIISOPROPANOLAMIDE (UNII: S485AM948Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50865-060-09 3785 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/11/2014 2 NDC:50865-060-27 1000 mL in 1 BAG; Type 0: Not a Combination Product 03/11/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 03/11/2014 Labeler - Kutol Products Company (004236139) Registrant - Kutol Products Company (004236139) Establishment Name Address ID/FEI Business Operations Kutol Products Company, Inc. 004236139 manufacture(50865-060)

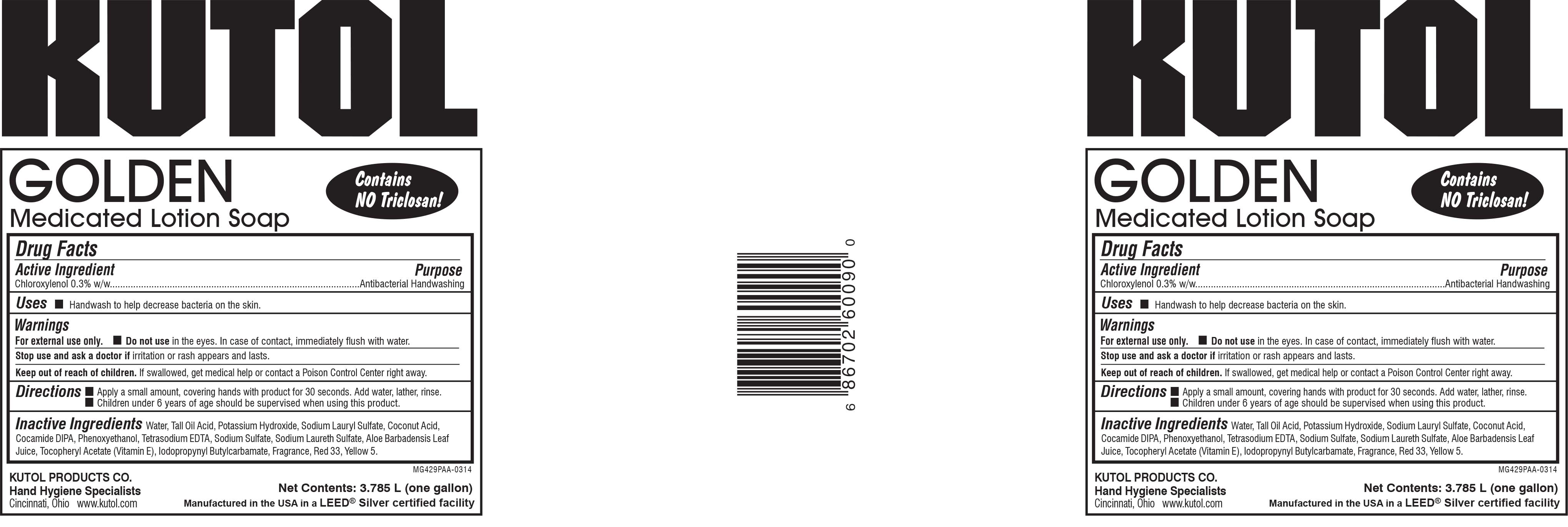

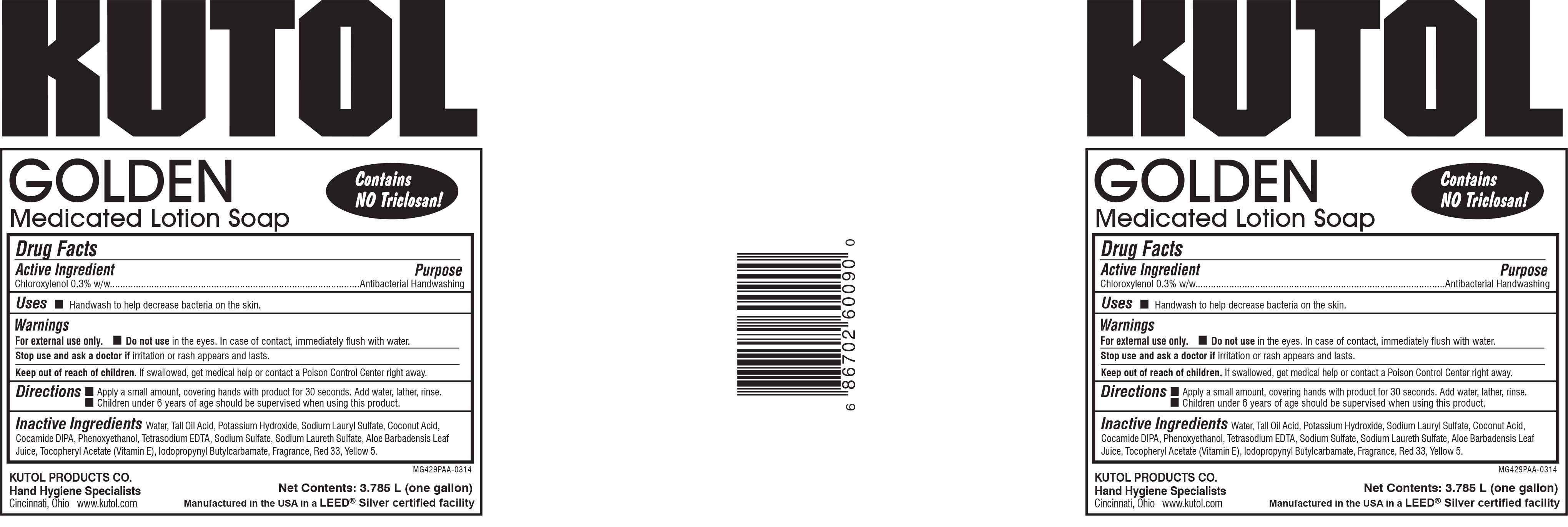 50865-060-09.jpg
50865-060-09.jpg
 50865-060-27.jpg
50865-060-27.jpg