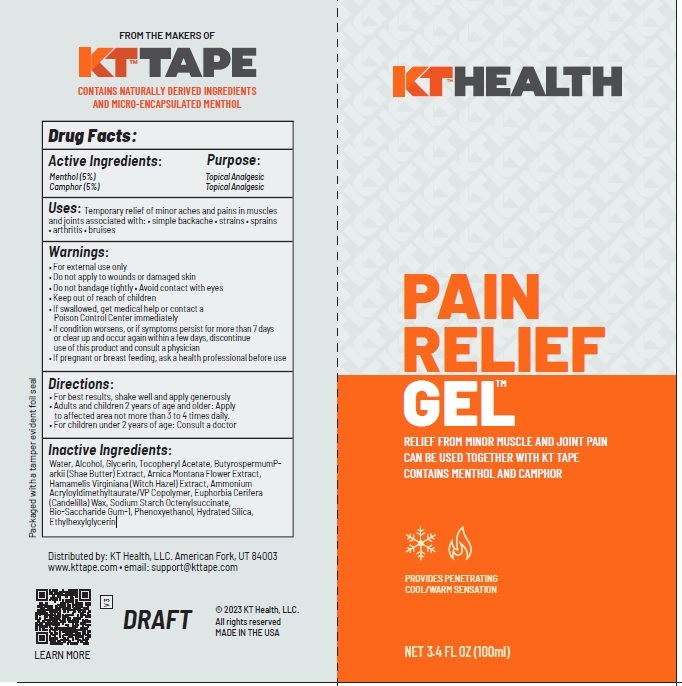
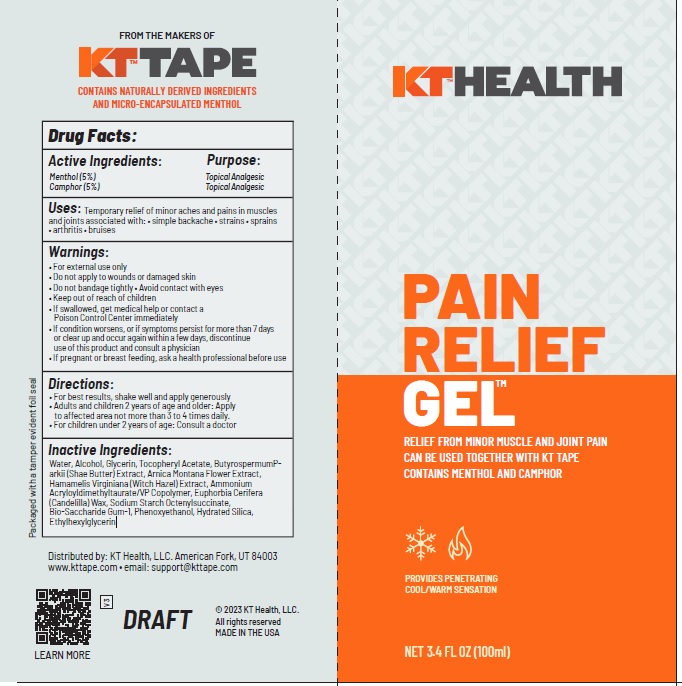
Label: KT RECOVERY PLUS PAIN RELIEF GEL- camphor, menthol gel
- NDC Code(s): 73044-103-01, 73044-103-02
- Packager: KT Health LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
Warnings
• For external use only
• Do not apply to wounds or damaged skin
• Do not bandage tightly • Avoid contact with eyes
• Keep out of reach of children
• If swallowed, get medical help or contact a Poison Control Center immediately
• If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a physician
• If pregnant or breast feeding, ask a health professional before use - KEEP OUT OF REACH OF CHILDREN
- Directions
-
INACTIVE INGREDIENT
Water, Alcohol, Glycerin, Tocopheryl Acetate, ButyrospermumParkii (Shae Butter) Extract, Arnica Montana Flower Extract, Hamamelis Virginiana (Witch Hazel) Extract, Ammonium Acryloyldimethyltaurate/VP Copolymer, Euphorbia Cerifera (Candelilla) Wax, Sodium Starch Octenylsuccinate,
Bio-Saccharide Gum-1, Phenoxyethanol, Hydrated Silica, Ethylhexylglycerin - Product label
-
INGREDIENTS AND APPEARANCE
KT RECOVERY PLUS PAIN RELIEF GEL
camphor, menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73044-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g in 100 mL CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SHEANUT (UNII: 84H6HBP32L) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) CANDELILLA WAX (UNII: WL0328HX19) OCTENYLSUCCINIC ACID (UNII: 12UZE4X73L) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDRATED SILICA (UNII: Y6O7T4G8P9) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73044-103-01 100 mL in 1 TUBE; Type 0: Not a Combination Product 04/15/2023 2 NDC:73044-103-02 1 in 1 BOX 04/15/2023 2 89 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/15/2023 Labeler - KT Health LLC (807008037) Establishment Name Address ID/FEI Business Operations Adonis Inc 116983147 manufacture(73044-103)