Label: SODIUM PHENYLACETATE AND SODIUM BENZOATE injection, solution, concentrate
- NDC Code(s): 72266-247-01
- Packager: FOSUN PHARMA USA INC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SODIUM PHENYLACETATE AND SODIUM BENZOATE INJECTION safely and effectively. See full prescribing information for SODIUM PHENYLACETATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESodium phenylacetate and sodium benzoate injection is indicated as adjunctive therapy in pediatric and adult patients for the treatment of acute hyperammonemia and associated encephalopathy in ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose - Sodium phenylacetate and sodium benzoate injection must be diluted with sterile 10% Dextrose Injection (D10W) before administration. The dilution and dosage of sodium ...
-
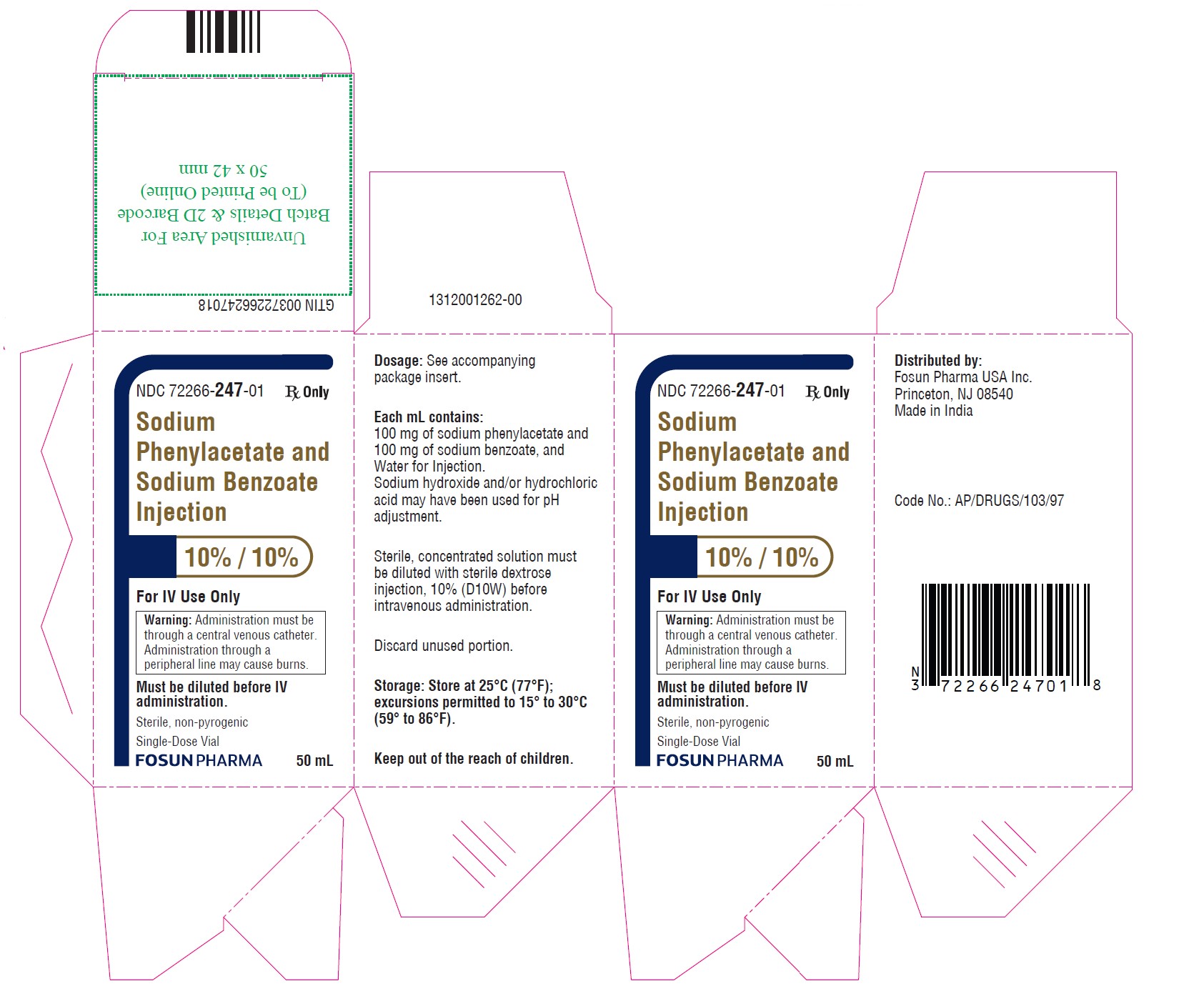
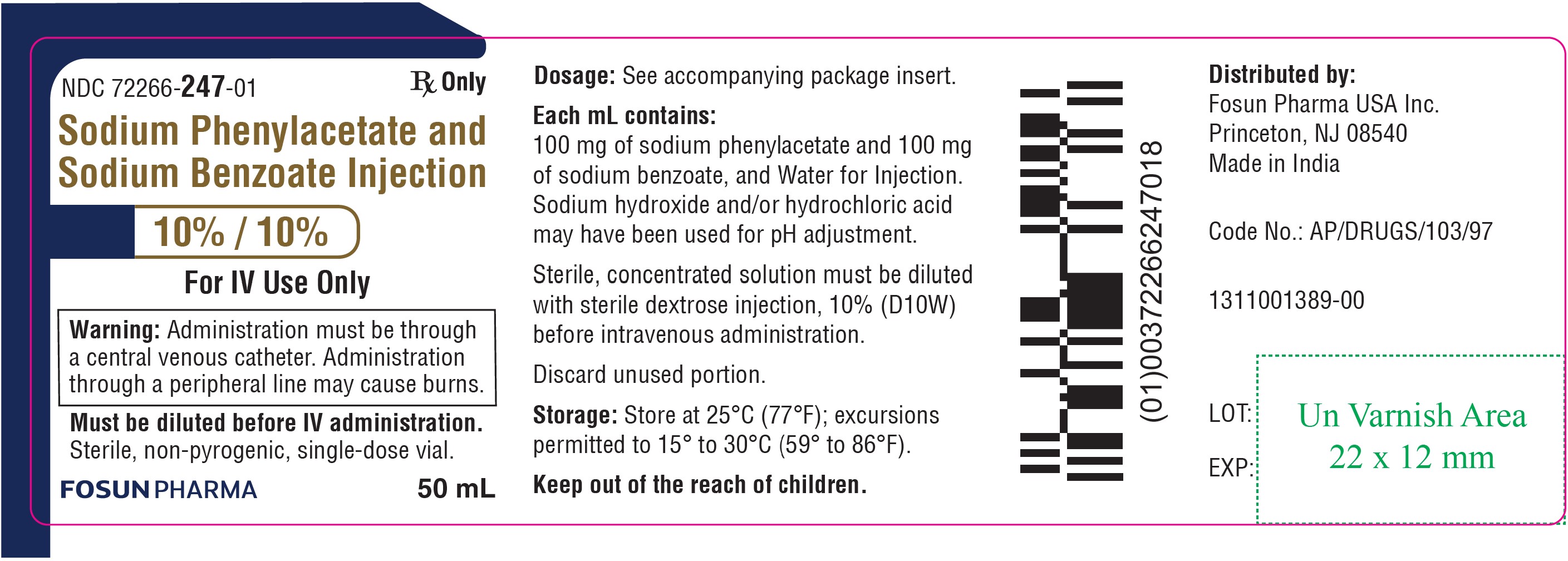
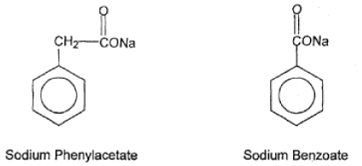
3 DOSAGE FORMS AND STRENGTHS Sodium Phenylacetate and Sodium Benzoate Injection, 10% per 10%, for intravenous use, is a sterile, concentrated, aqueous solution of sodium phenylacetate and sodium benzoate.
-
4 CONTRAINDICATIONS None.
-
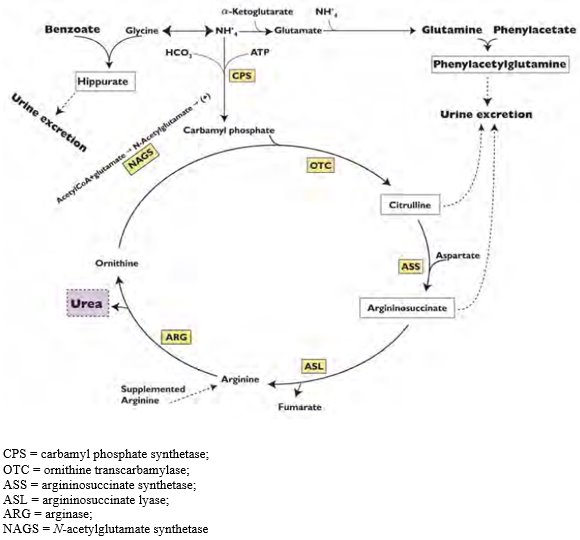
5 WARNINGS AND PRECAUTIONS 5.1 Decreased Potassium Levels - Because urine potassium loss is enhanced by the excretion of the non‑reabsorbable anions, phenylacetylglutamine and hippurate, plasma potassium levels should be ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS Formal drug interaction studies have not been performed with sodium phenylacetate and sodium benzoate injection. Some antibiotics such as penicillin may compete with phenylacetylglutamine and ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Available data with sodium phenylacetate and sodium benzoate injection use in pregnant women are insufficient to identify a drug-associated risk of major birth ...
-
10 OVERDOSAGE Overdosage has been reported during sodium phenylacetate and sodium benzoate injection treatment in urea cycle-deficient patients. All patients in the uncontrolled open-label study were to be ...
-
11 DESCRIPTION Sodium phenylacetate and sodium benzoate injection, 10% per 10% (a nitrogen binding agent) is a sterile, concentrated, aqueous solution of sodium phenylacetate and sodium benzoate. The pH of the ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Urea cycle disorders can result from decreased activity of any of the following enzymes: N-acetylglutamate synthetase (NAGS), carbamyl phosphate ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate the carcinogenic potential of sodium phenylacetate and sodium ...
-
14 CLINICAL STUDIES The efficacy of sodium phenylacetate and sodium benzoate injection in improving patient survival of acute hyperammonemic episodes was demonstrated in an analysis of 316 patients (1,045 episodes of ...
-
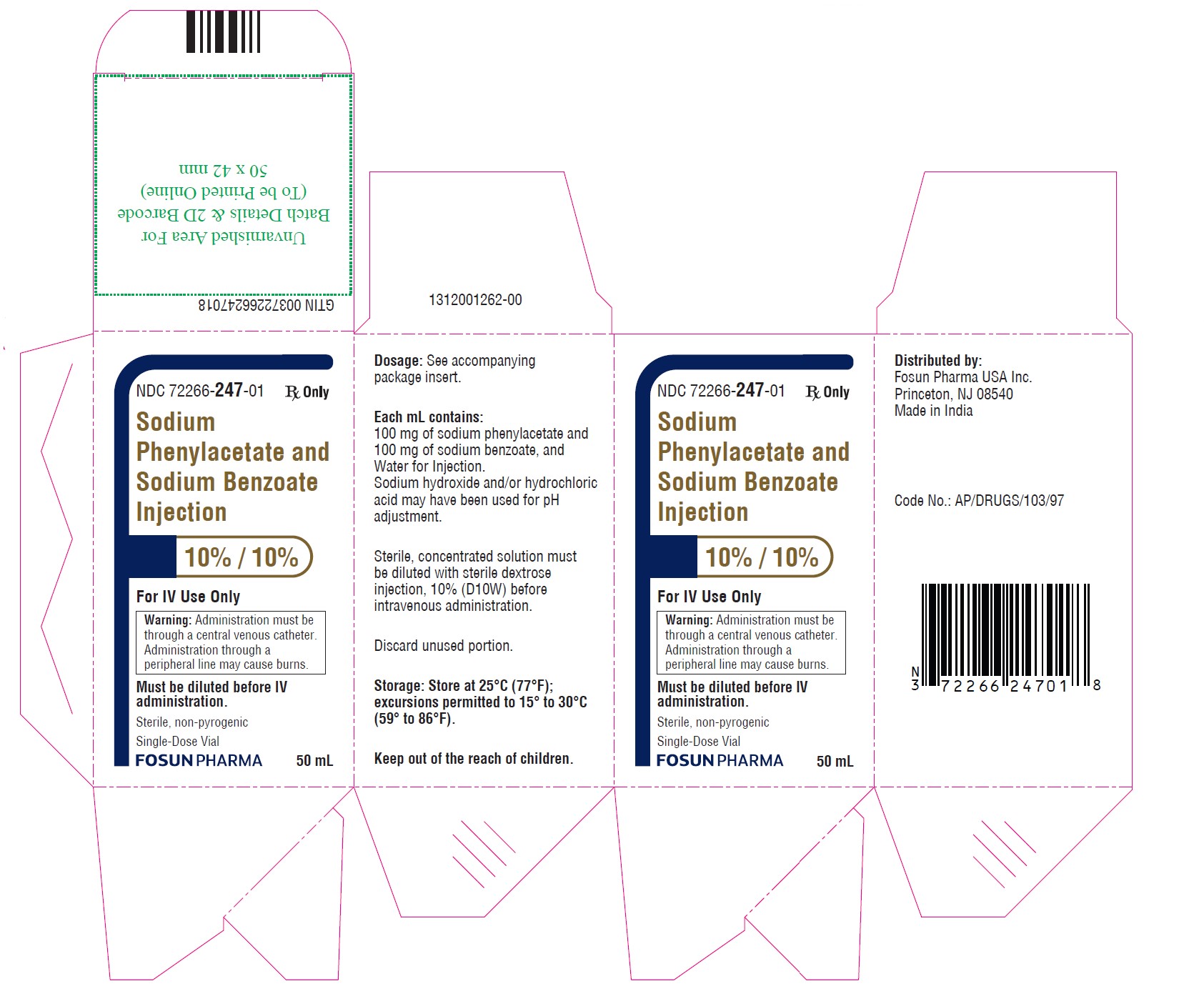
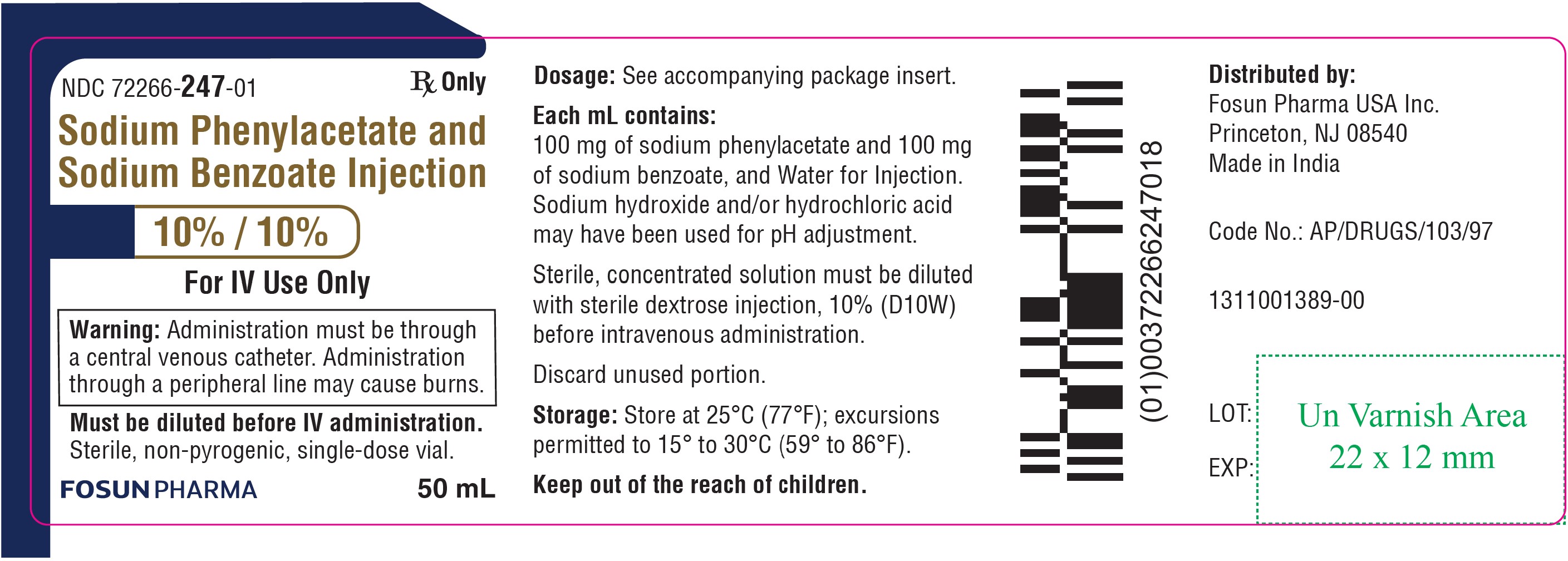
16 HOW SUPPLIED/STORAGE AND HANDLINGSodium Phenylacetate and Sodium Benzoate Injection, 10% per 10% is supplied in a sterile, non-pyrogenic, single-dose glass vial. NDC 72266-247-01 - single-dose vial containing 50 mL of ...
-
17 PATIENT COUNSELING INFORMATIONPhysicians should advise patients and caregivers about the following for safe use of sodium phenylacetate and sodium benzoate injection: When plasma ammonia levels have normalized, dietary ...
-
PRINCIPAL DISPLAY PANEL – 10%/10%NDC 72266-247-01 50 mL - Sodium Phenylacetate and Sodium Benzoate Injection 10% / 10% For IV Use Only - Warning: Administration must be through a central venous catheter ...
-
INGREDIENTS AND APPEARANCEProduct Information