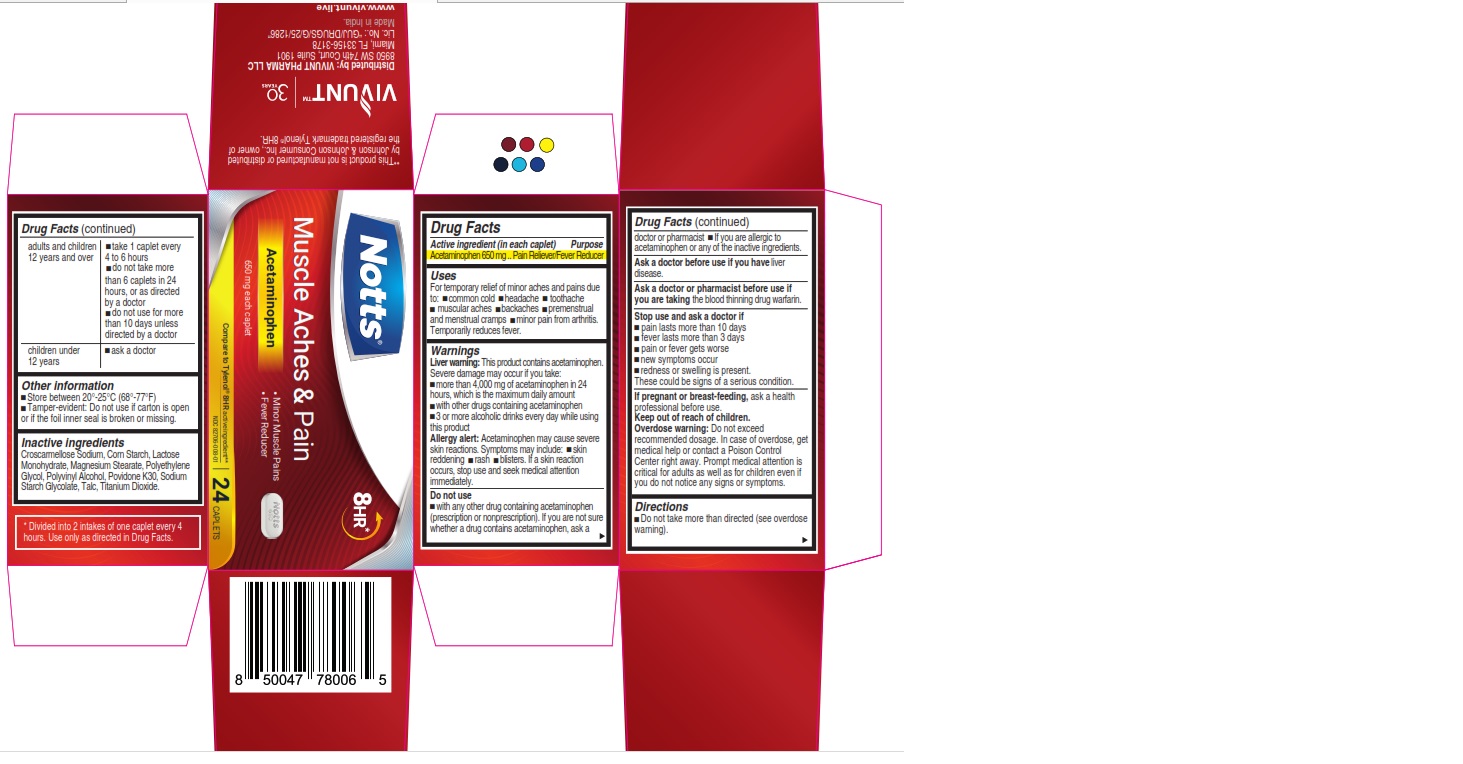
Label: NOTTS - MUSCLE ACHES AND PAIN- acetaminophen tablet
- NDC Code(s): 82706-008-01, 82706-008-02
- Packager: VIVUNT PHARMA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Liver Warning
This product contains acetaminophen. Severe damage may occur if you take:
● more than 4,000 mg of acetaminophen in 24 hours, which isthe maximum daily amount.
● with other drugs containing acetaminophen
● 3 or more alcoholic drinks every day while using this productAllergy Alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- rash
- blisters
If a skin reaction occurs, stop use and seek medical attention immediately.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients.
- Directions
- Other information
- Inactive ingredients
-
SPL UNCLASSIFIED SECTION
* Divided into 2 intakes of one caplet every 4 hours. Use only as directed in Drug Facts.
**This product is not manufactured or distributed by Johnson & Johnson Consumer Inc., owner of the registered trademark Tylenol® 8HR.Distributed by:
VIVUNT PHARMA LLC
8950 SW 74th Court, Suite 1901
Miami, Florida FL 33156-3178
Made in India
- PRINCIPAL DISPLAY PANEL - 24 Caplets
- PRINCIPAL DISPLAY PANEL - 100 Caplets
-
INGREDIENTS AND APPEARANCE
NOTTS - MUSCLE ACHES AND PAIN
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82706-008 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white Score no score Shape OVAL Size 16mm Flavor Imprint Code Notts;650 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82706-008-02 1 in 1 CARTON 08/17/2022 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:82706-008-01 1 in 1 CARTON 08/17/2022 2 24 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 08/17/2022 Labeler - VIVUNT PHARMA LLC (045829437)