Label: NOREPINEPHRINE BITARTRATE injection
- NDC Code(s): 64980-630-04, 64980-630-41
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NOREPINEPHRINE BITARTRATE INJECTION safely and effectively. See full prescribing information for NOREPINEPHRINE BITARTRATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENorepinephrine Bitartrate Injection is indicated to raise blood pressure in adult patients with severe, acute hypotension.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Instructions - Correct Hypovolemia - Address hypovolemia before initiation of Norepinephrine Bitartrate Injection therapy. If the patient does not respond ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 4 mg/4 mL (1 mg/mL norepinephrine base) sterile, clear colorless liquid intended for intravenous infusion only in a single-dose amber glass vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Tissue Ischemia - Administration of Norepinephrine Bitartrate Injection to patients who are hypotensive from hypovolemia can result in severe peripheral and visceral vasoconstriction ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in greater detail in other sections: Tissue Ischemia [see Warnings and Precautions (5.1)] Hypotension [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 MAO-Inhibiting Drugs - Co-administration of Norepinephrine Bitartrate Injection with monoamine oxidase (MAO) inhibitors or other drugs with MAO-inhibiting properties (e.g., linezolid) can ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited published data consisting of a small number of case reports and multiple small trials involving the use of norepinephrine in pregnant women at the time of ...
-
10 OVERDOSAGEOverdosage with Norepinephrine Bitartrate Injection may result in headache, severe hypertension, reflex bradycardia, marked increase in peripheral resistance, and decreased cardiac output. In ...
-
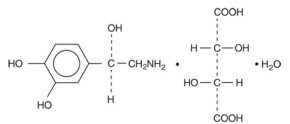
11 DESCRIPTIONNorepinephrine (sometimes referred to as l-arterenol/Levarterenol or l-norepinephrine) is a sympathomimetic amine which differs from epinephrine by the absence of a methyl group on the nitrogen ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Norepinephrine is a peripheral vasoconstrictor (alpha-adrenergic action) and an inotropic stimulator of the heart and dilator of coronary arteries (beta-adrenergic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis, mutagenesis, and fertility studies have not been performed.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNorepinephrine Bitartrate Injection, USP is a sterile, clear colorless solution for injection intended for intravenous use. It contains the equivalent of 1 mg of norepinephrine base per 1 mL (4 ...
-
17 PATIENT COUNSELING INFORMATIONRisk of Tissue Damage - Advise the patient, family, or caregiver to report signs of extravasation urgently [see Warnings and Precautions (5.1)]. Rx only - Manufactured for: Rising Pharma ...
-
PRINCIPAL DISPLAY PANEL - 4 mL Vial LabelNDC 64980-630-04 - Rx only - Norepinephrine - Bitartrate Injection, USP - 4 mg/4mL (1mg/mL) FOR INTRAVENOUS INFUSION ONLY. DILUTE BEFORE USE. PROTECT FROM LIGHT - Warning: Contains Sulfites. 4 mL ...
-
PRINCIPAL DISPLAY PANEL - 4 mL Carton LabelNDC 64980-630-41 - Sterile Injection - Rx only - Norepinephrine - Bitartrate Injection, USP - 4 mg/4 mL (1 mg/mL) FOR INTRAVENOUS INFUSION ONLY. Warning: This is a potent drug. Dosage should be controlled ...
-
INGREDIENTS AND APPEARANCEProduct Information