Label: MESALAMINE capsule, extended release
- NDC Code(s): 60687-650-32, 60687-650-33
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 67877-717
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MESALAMINE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for MESALAMINE EXTENDED-RELEASE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMesalamine extended-release capsules are indicated for the maintenance of remission of ulcerative colitis in adults.
-
2 DOSAGE AND ADMINISTRATIONDosage - The recommended dosage in adults is 1.5 g (four 0.375 g capsules) orally once daily in the morning. Administration Instructions - Evaluate renal function before initiating therapy ...
-
3 DOSAGE FORMS AND STRENGTHSExtended-release capsules: 0.375 g mesalamine in a light blue opaque, size “00” hard gelatin capsules filled with off- white to tan colored pellets. The capsules are imprinted with “MES” on cap ...
-
4 CONTRAINDICATIONSMesalamine extended-release capsules are contraindicated in patients with hypersensitivity to salicylates or aminosalicylates or to any of the components of mesalamine extended-release capsules ...
-
5 WARNINGS AND PRECAUTIONS5.1 Renal Impairment - Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and renal failure, has been reported in patients given products such as ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: Renal Impairment - [see - Warnings and Precautions (5.1)] Mesalamine-Induced Acute ...
-
7 DRUG INTERACTIONS7.1 Antacids - Because the dissolution of the coating of the granules in mesalamine extended-release capsules depends on pH, avoid co-administration of mesalamine extended-release capsules with ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Published data from meta-analyses, cohort studies and case series on the use of mesalamine during pregnancy have not reliably informed an association with ...
-
10 OVERDOSAGEMesalamine extended-release capsules are an aminosalicylate, and symptoms of salicylate toxicity include nausea, vomiting and abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic ...
-
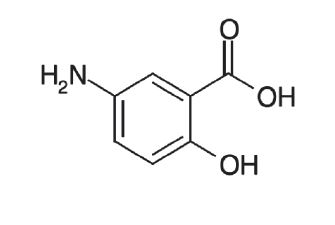
11 DESCRIPTIONEach mesalamine extended-release capsule is a delayed-and extended-release dosage form for oral administration. Each capsule contains 0.375 g of mesalamine USP (5-aminosalicylic acid, 5-ASA), an ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of mesalamine (5-ASA) is not fully understood, but appears to be local anti-inflammatory effect on colonic epithelial cells. Mucosal production ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Dietary mesalamine was not carcinogenic in rats at doses as high as 480 mg/kg/day, or in mice at 2,000 mg/kg/day. These doses are ...
-
14 CLINICAL STUDIESTwo similar, randomized, double-blind, placebo-controlled, multi-center studies were conducted in a total of 562 adult patients in remission from ulcerative colitis. The study populations had a ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMesalamine extended-release capsules, USP are available as light blue opaque, size “00” hard gelatin capsules filled with off- white to tan colored pellets. The capsules are imprinted with “MES ...
-
17 PATIENT COUNSELING INFORMATIONAdministration - Instruct patients: Swallow the capsules whole. Do not cut, break, crush or chew the capsules. Avoid co-administration of mesalamine extended-release capsules with ...
-
PACKAGING INFORMATIONAmerican Health Packaging unit dose blisters (see - How Supplied section) contain drug product from Ascend Laboratories, LLC as follows: (0.375 g / 20 UD) NDC 60687-650-32 packaged from NDC ...
-
Package/Label Display Panel – Carton – 0.375 gNDC 60687- 650-32 - Mesalamine - Extended-Release - Capsules, USP - 0.375 g - 20 Capsules (5 x 4) Rx Only - Each Capsule Contains: Mesalamine ...
-
Package/Label Display Panel – Blister – 0.375 gMesalamine - Extended-Release - Capsule, USP - 0.375 g
-
INGREDIENTS AND APPEARANCEProduct Information