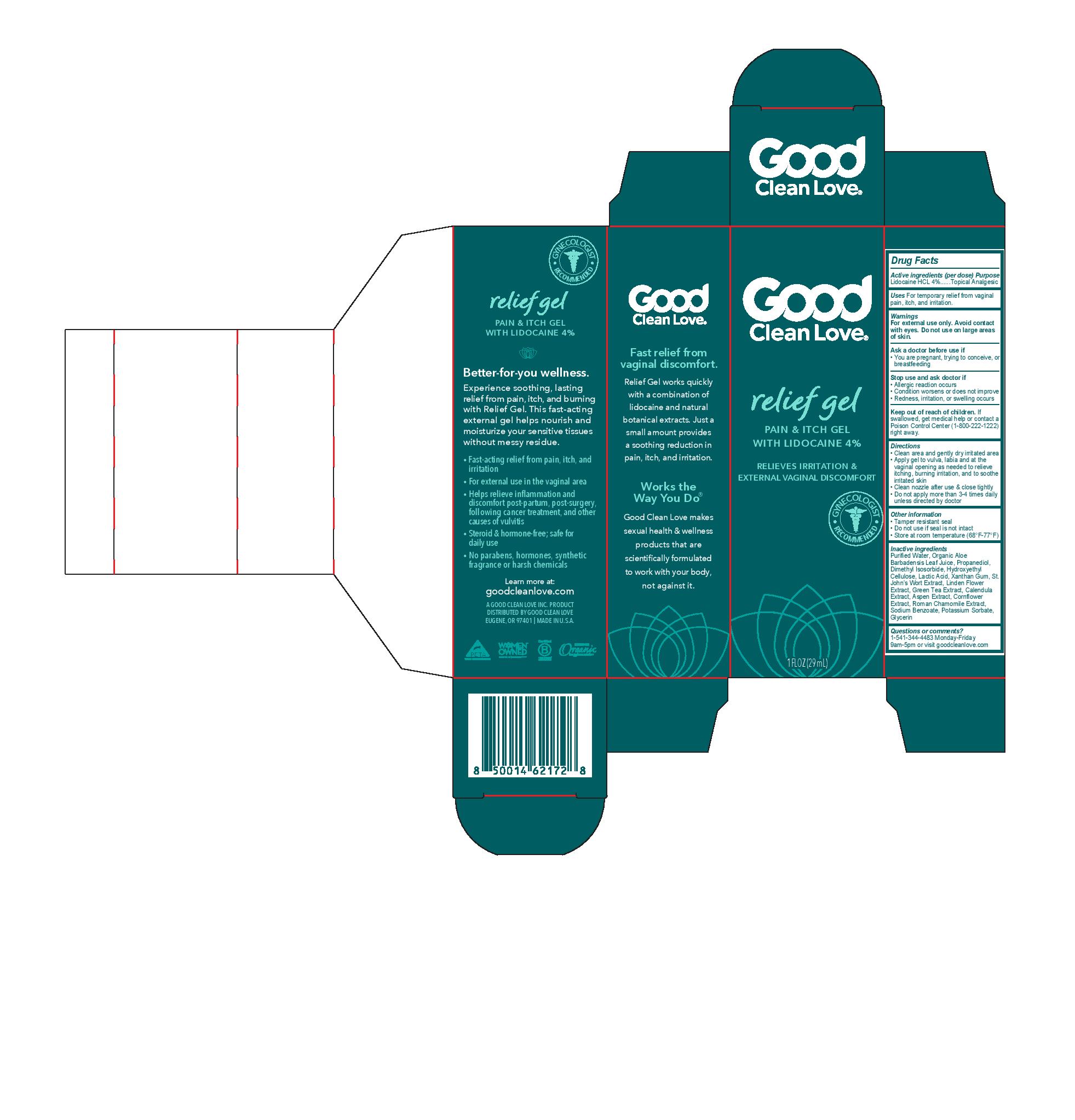
Label: RELIEF GEL- lidocaine hcl gel
- NDC Code(s): 73716-003-05, 73716-003-29
- Packager: Good Clean Love, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (per dose)
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Purified Water, Organic Aloe Barbadensis Leaf Juice, Propanediol, Dimethyl Isosorbide, Hydroxyethyl Cellulose, Lactic Acid, Xanthan Gum, St. John’s Wort Extract, Linden Flower Extract, Green Tea Extract, Calendula Extract, Aspen Extract, Cornflower Extract, Roman Chamomile Extract, Sodium Benzoate, Potassium Sorbate, Glycerin
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RELIEF GEL
lidocaine hcl gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73716-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPANEDIOL (UNII: 5965N8W85T) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) ST. JOHN'S WORT (UNII: UFH8805FKA) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) TILIA CORDATA FLOWER (UNII: CFN6G1F6YK) POPULUS TREMULOIDES BARK (UNII: 5543O0CEID) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) LACTIC ACID (UNII: 33X04XA5AT) XANTHAN GUM (UNII: TTV12P4NEE) CENTAUREA CYANUS FLOWER (UNII: QZ239038YC) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73716-003-29 1 in 1 CARTON 04/08/2022 1 29 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:73716-003-05 5 mL in 1 POUCH; Type 0: Not a Combination Product 04/08/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/08/2022 Labeler - Good Clean Love, Inc. (153909531) Establishment Name Address ID/FEI Business Operations TRI-PAC, INC. 020844956 manufacture(73716-003)