Label: ONDANSETRON solution
- NDC Code(s): 17856-0691-1, 17856-0691-2, 17856-0691-4, 17856-0691-5, view more
- Packager: ATLANTIC BIOLOGICALS CORP.
- This is a repackaged label.
- Source NDC Code(s): 65162-691
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONONDANSETRON ORAL SOLUTION - HIGHLIGHTS OF PRESCRIBING INFORMATION - These highlights do not include all the information needed to use ONDANSETRON ORAL SOLUTION safely and effectively. See full ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEOndansetron oral solution is indicated for the prevention of nausea and vomiting associated with: highly emetogenic cancer chemotherapy, including cisplatin greater than or equal to 50 mg/m ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage - The recommended dosage regimens for adult and pediatric patients are described in Table 1 and Table 2, respectively. Corresponding doses of ondansetron oral solution may be used ...
-
3 DOSAGE FORMS AND STRENGTHSOndansetron oral solution, USP, 4 mg/5 mL, is a clear, colorless liquid with a characteristic strawberry odor available in a 50-mL bottle.
-
4 CONTRAINDICATIONSOndansetron is contraindicated in patients: known to have hypersensitivity (e.g., anaphylaxis) to ondansetron or any of the components of the formulation - [see - Adverse Reactions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including anaphylaxis and bronchospasm, have been reported in patients who have exhibited hypersensitivity to other selective 5-HT3 ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Serotonergic Drugs - Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data do not reliably inform the association of ondansetron and adverse fetal outcomes. Published epidemiological studies on the association between ...
-
9 DRUG ABUSE AND DEPENDENCEAnimal studies have shown that ondansetron is not discriminated as a benzodiazepine nor does it substitute for benzodiazepines in direct addiction studies.
-
10 OVERDOSAGEThere is no specific antidote for ondansetron overdose. Patients should be managed with appropriate supportive therapy. In addition to the adverse reactions listed above, the following adverse ...
-
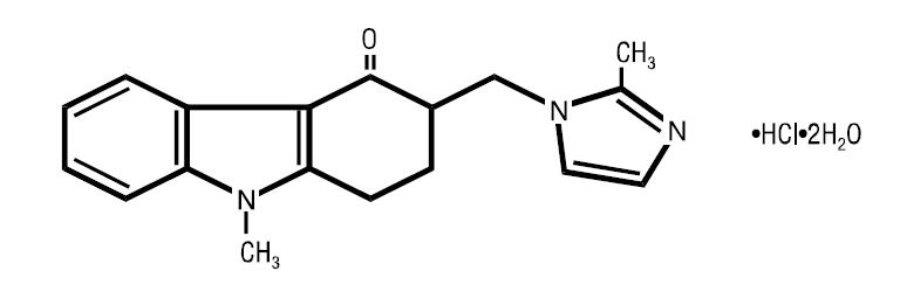
11 DESCRIPTIONThe active ingredient in ondansetron oral solution, USP is ondansetron hydrochloride, USP as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ondansetron is a selective 5-HT - 3receptor antagonist. While its mechanism of action has not been fully characterized, ondansetron is not a dopamine-receptor ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis and Mutagenesis, Impairment of Fertility - Carcinogenic effects were not seen in 2-year studies in rats and mice with oral ondansetron doses up to 10 mg/kg per day and 30 ...
-
14 CLINICAL STUDIES14.1 Prevention of Chemotherapy-induced Nausea and Vomiting - Highly Emetogenic Chemotherapy - In two randomized, double-blind, monotherapy trials, a single 24-mg oral dose of ondansetron was ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Product: 17856-0691 - NDC: 17856-0691-5 5 mL in a CUP - NDC: 17856-0691-1 1.5 mL in a SYRINGE - NDC: 17856-0691-2 2.5 mL in a SYRINGE, PLASTIC
-
17 PATIENT COUNSELING INFORMATIONQT Prolongation - Inform patients that ondansetron may cause serious cardiac arrhythmias such as QT prolongation. Instruct patients to tell their healthcare provider right away if they perceive a ...
-
ONDANSETRON SOLUTIONONDANSETRON ORAL SOL - RX ONLY
-
INGREDIENTS AND APPEARANCEProduct Information