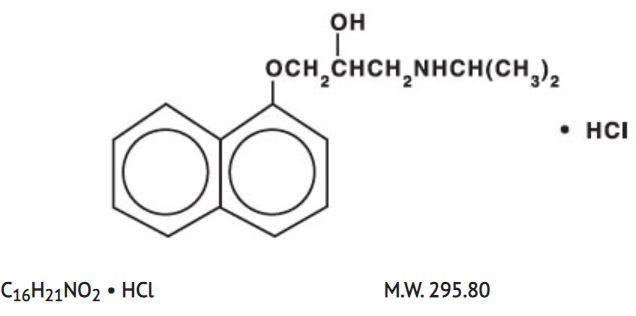
General
-
Propranolol is a nonselective beta-adrenergic receptor blocking agent possessing no other autonomic nervous system activity. It specifically competes with beta-adrenergic receptor ...
General
Propranolol is a nonselective beta-adrenergic receptor blocking agent possessing no other autonomic nervous system activity. It specifically competes with beta-adrenergic receptor stimulating agents for available receptor sites. When access to beta-receptor sites is blocked by propranolol, chronotropic, inotropic, and vasodilator responses to beta-adrenergic stimulation are decreased proportionately.
At doses greater than required for beta-blockade, propranolol also exerts a quinidine-like or anesthetic-like membrane action, which affects the cardiac action potential. The significance of the membrane action in the treatment of arrhythmias is uncertain.
Mechanism of Action
The effects of propranolol are due to selective blockade of beta-adrenergic receptors, leaving alpha-adrenergic responses intact. There are two well-characterized subtypes of beta receptors (beta1 and beta2); propranolol interacts with both subtypes equally. Beta1-adrenergic receptors are found primarily in the heart. Blockade of cardiac beta1-adrenergic receptors leads to a decrease in the activity of both normal and ectopic pacemaker cells and a decrease in A-V nodal conduction velocity. All of these actions can contribute to antiarrhythmic activity and control of ventricular rate during arrhythmias. Blockade of cardiac beta1-adrenergic receptors also decreases the myocardial force of contraction and may provoke cardiac decompensation in patients with minimal cardiac reserve.
Beta2-adrenergic receptors are found predominantly in smooth muscle-vascular, bronchial, gastrointestinal and genitourinary. Blockade of these receptors results in constriction. Clinically, propranolol may exacerbate respiratory symptoms in patients with obstructive pulmonary diseases such as asthma and emphysema (see CONTRAINDICATIONS and WARNINGS).
Propranolol’s beta-blocking effects are attributable to its S(-) enantiomer.
Pharmacokinetics and Drug Mechanism
Distribution
Propranolol has a distribution half-life (T1/2 alpha) of 5 to 10 minutes and a volume of distribution of about 4 to 5 L/kg. Approximately 90% of circulating propranolol is bound to plasma proteins. The binding is enantiomer-selective. The S-isomer is preferentially bound to alpha1 glycoprotein and the R-isomer is preferentially bound to albumin.
Metabolism and Elimination
The elimination half-life (T1/2 alpha) is between 2 and 5.5 hours. Propranolol is extensively metabolized with most metabolites appearing in the urine. The major metabolites include propranolol glucuronide, naphthyloxylactic acid, and glucuronic acid and sulfate conjugates of 4-hydroxy propranolol. Following single-dose intravenous administration, side-chain oxidative products account for approximately 40% of the metabolites, direct conjugation products account for approximately 45 to 50% of metabolites, and ring oxidative products account for approximately 10 to 15% of metabolites. Of these, only the primary ring oxidative product (4-hydroxypropranolol) possesses beta-adrenergic receptor blocking activity.
In vitro studies have indicated that the aromatic hydroxylation of propranolol is catalyzed mainly by polymorphic CYP2D6. Side-chain oxidation is mediated mainly by CYP1A2 and to some extent by CYP2D6. 4-hydroxy propranolol is a weak inhibitor of CYP2D6.
Pharmacodynamics
As propranolol concentration increases, so does its beta-blocking effect, as evidenced by a reduction in exercise-induced tachycardia (n=6 normal volunteers).
Special Populations
Pediatric
The pharmacokinetics of propranolol have not been investigated in patients under 18 years of age. Propranolol injection is not recommended for treatment of cardiac arrhythmias in pediatric patients.
Geriatric
Elevated propranolol plasma concentrations, a longer mean elimination half-life (254 vs. 152 minutes), and decreased systemic clearance (8 vs. 13 mL/kg/min) have been observed in elderly subjects when compared to young subjects. However, the apparent volume of distribution seems to be similar in elderly and young subjects. These findings suggest that dose adjustment of propranolol injection may be required for elderly patients (see PRECAUTIONS).
Gender
Intravenously administered propranolol was evaluated in 5 women and 6 men. When adjusted for weight, there were no gender-related differences in elimination half-life, volume of distribution, protein binding, or systemic clearance.
Obesity
In a study of intravenously administered propranolol, obese subjects had a higher AUC (161 versus 109 hr•mcg/L) and lower total clearance than did non-obese subjects. Propranolol plasma protein binding was similar in both groups.
Renal Insufficiency
The pharmacokinetics of propranolol and its metabolites were evaluated in 15 subjects with varying degrees of renal function after propranolol administration via the intravenous and oral routes. When compared with normal subjects, an increase in fecal excretion of propranolol conjugates was observed in patients with increased renal impairment. Propranolol was also evaluated in 5 patients with chronic renal failure, 6 patients on regular dialysis, and 5 healthy subjects, following a single oral dose of 40 mg of propranolol. The peak plasma concentrations (Cmax) of propranolol in the chronic renal failure group were 2- to 3-fold higher (161 ng/mL) than those observed in the dialysis patients (47 ng/mL) and in the healthy subjects (26 ng/mL). Propranolol plasma clearance was also reduced in the patients with chronic renal failure.
Chronic renal failure has been associated with a decrease in drug metabolism via downregulation of hepatic cytochrome P450 activity.
Hepatic Insufficiency
Propranolol is extensively metabolized by the liver. In a study conducted in 6 normal subjects and 20 patients with chronic liver disease, including hepatic cirrhosis, 40 mg of R-propranolol was administered intravenously. Compared to normal subjects, patients with chronic liver disease had decreased clearance of propranolol, increased volume of distribution, decreased protein-binding, and considerable variation in half-life. Caution should be exercised when propranolol is used in this population. Consideration should be given to lowering the dose of intravenous propranolol in patients with hepatic insufficiency (see PRECAUTIONS).
Thyroid Dysfunction
No pharmacokinetic changes were observed in hyperthyroid or hypothyroid patients when compared to their corresponding euthyroid state. Dosage adjustment does not seem necessary in either patient population based on pharmacokinetic findings.
Drug Interactions
Interactions with Substrates, Inhibitors or Inducers of Cytochrome P-450 Enzymes
Because propranolol’s metabolism involves multiple pathways in the cytochrome P-450 system (CYP2D6, 1A2, 2C19), administration of propranolol with drugs that are metabolized by, or affect the activity (induction or inhibition) of one or more of these pathways may lead to clinically relevant drug interactions (see PRECAUTIONS, DRUG INTERACTIONS).
Substrates or Inhibitors of CYP2D6
Blood levels of propranolol may be increased by administration of propranolol with substrates or inhibitors of CYP2D6, such as amiodarone, cimetidine, delavirdine, fluoxetine, paroxetine, quinidine, and ritonavir. No interactions were observed with either ranitidine or lansoprazole.
Substrates or Inhibitors of CYP1A2
Blood levels of propranolol may be increased by administration of propranolol with substrates or inhibitors of CYP1A2, such as imipramine, cimetidine, ciprofloxacin, fluvoxamine, isoniazid, ritonavir, theophylline, zileuton, zolmitriptan, and rizatriptan.
Substrates or Inhibitors of CYP2C19
Blood levels of propranolol may be increased by administration of propranolol with substrates or inhibitors of CYP2C19, such as fluconazole, cimetidine, fluoxetine, fluvoxamine, teniposide, and tolbutamide. No interaction was observed with omeprazole.
Inducers of Hepatic Drug Metabolism
Blood levels of propranolol may be decreased by administration of propranolol with inducers such as rifampin and ethanol. Cigarette smoking also induces hepatic metabolism and has been shown to increase up to 100% the clearance of propranolol, resulting in decreased plasma concentrations.
Cardiovascular Drugs
Antiarrhythmics
The AUC of propafenone is increased by more than 200% with coadministration of propranolol.
The metabolism of propranolol is reduced by coadministration of quinidine, leading to a 2- to 3- fold increased blood concentrations and greater beta-blockade.
The metabolism of lidocaine is inhibited by coadministration of propranolol, resulting in a 25% increase in lidocaine concentrations.
Calcium Channel Blockers
The mean Cmax and AUC of propranolol are increased respectively, by 50% and 30% by coadministration of nisoldipine and by 80% and 47%, by coadministration of nicardipine.
The mean values of Cmax and AUC of nifedipine are increased by 64% and 79%, respectively, by coadministration of propranolol.
Propranolol does not affect the pharmacokinetics of verapamil and norverapamil. Verapamil does not affect the pharmacokinetics of propranolol.
Non-Cardiovascular Drugs
Migraine Drugs
Administration of zolmitriptan or rizatriptan with propranolol resulted in increased concentrations of zolmitriptan (AUC increased by 56% and Cmax by 37%) or rizatriptan (the AUC and Cmax were increased by 67% and 75%, respectively).
Theophylline
Coadministration of theophylline with propranolol decreases theophylline clearance by 33% to 52%.
Benzodiazepines
Propranolol can inhibit the metabolism of diazepam, resulting in increased concentrations of diazepam and its metabolites. Diazepam does not alter the pharmacokinetics of propranolol.
The pharmacokinetics of oxazepam, triazolam, lorazepam, and alprazolam are not affected by coadministration of propranolol.
Neuroleptic Drugs
Coadministration of propranolol at doses greater than or equal to 160 mg/day resulted in increased thioridazine plasma concentrations ranging from 50% to 370% and increased thioridazine metabolites concentrations ranging from 33% to 210%.
Coadministration of chlorpromazine with propranolol resulted in increased plasma levels of both drugs (70% increase in propranolol concentrations).
Anti-Ulcer Drugs
Coadministration of propranolol with cimetidine, a non-specific CYP450 inhibitor, increased propranolol concentrations by about 40%. Coadministration with aluminum hydroxide gel (1200 mg) resulted in a 50% decrease in propranolol concentrations.
Coadministration of metoclopramide with propranolol did not have a significant effect on propranolol’s pharmacokinetics.
Lipid Lowering Drugs
Coadministration of cholesteramine or colestipol with propranolol resulted in up to 50% decrease in propranolol concentrations.
Coadministration of propranolol with lovastatin or pravastatin decreased 20% to 25% the AUC of both, but did not alter their pharmacodynamics. Propranolol did not have an effect on the pharmacokinetics of fluvastatin.
Warfarin
Concomitant administration of propranolol and warfarin has been shown to increase warfarin bioavailability and increase prothrombin time.
Close