Label: PHENTERMINE HYDROCHLORIDE capsule
- NDC Code(s): 72789-287-14, 72789-287-28, 72789-287-30
- Packager: PD-Rx Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 11534-176
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Phentermine Hydrochloride Capsules USP safely and effectively. See full prescribing information for Phentermine Hydrochloride ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Phentermine hydrochloride capsules are indicated as a short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the ...
-
2 DOSAGE AND ADMINISTRATION
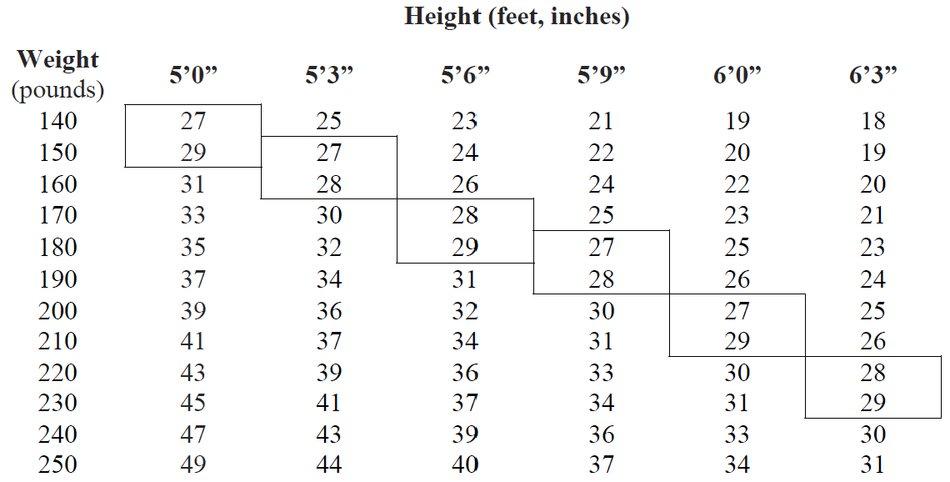
2.1 Exogenous Obesity - Dosage should be individualized to obtain an adequate response with the lowest effective dose. The usual adult dose is 15 mg to 30 mg as prescribed by the ...
-
3 DOSAGE FORMS AND STRENGTHS
Capsules containing 15 mg or 30 mg phentermine hydrochloride (equivalent to 12 mg or 24 mg phentermine base, respectively). 15 mg capsules: gray opaque cap, yellow opaque body with black imprint ...
-
4 CONTRAINDICATIONS
• History of cardiovascular disease (e.g., coronary artery disease, stroke, arrhythmias, congestive heart failure, uncontrolled hypertension) • During or within 14 days following the ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Co-administration with Other Drug Products for Weight Loss - Phentermine hydrochloride capsules are indicated only as short-term (a few weeks) monotherapy for the management of exogenous ...
-
6 ADVERSE REACTIONS
The following adverse reactions are described, or described in greater detail, in other sections: • Primary pulmonary hypertension [see - WARNINGS AND PRECAUTIONS ( 5.2) ...
-
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase Inhibitors - Use of phentermine is contraindicated during or within 14 days following the administration of monoamine oxidase inhibitors because of the risk of ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy categoryX - Phentermine is contraindicated during pregnancy because weight loss offers no potential benefit to a pregnant woman and may result in fetal harm. A ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - Phentermine is a Schedule IV controlled substance. 9.2 Abuse - Phentermine is related chemically and pharmacologically to the amphetamines. Amphetamines and ...
-
10 OVERDOSAGE
The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage. 10.1 Acute Overdosage - Manifestations of acute overdosage include ...
-
11 DESCRIPTION
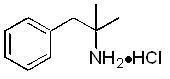
Phentermine hydrochloride is a sympathomimetic amine anorectic. Its chemical name is α,α, dimethylphenethylamine hydrochloride. The structural formula is as follows: C - 10H ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Phentermine is a sympathomimetic amine with pharmacologic activity similar to the prototype drugs of this class used in obesity, amphetamine (d- and d ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies have not been performed with phentermine to determine the potential for carcinogenesis, mutagenesis or impairment of ...
-
14 CLINICAL STUDIES
In relatively short-term clinical trials, adult obese subjects instructed in dietary management and treated with “anorectic” drugs lost more weight on the average than those treated with placebo ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Phentermine hydrochloride capsules USP, for oral administration, are available as: 30 mg: Blue/Clear powder filled capsules; imprinted “N16” in black ink and supplied as: NDC 72789-287-14 ...
-
17 PATIENT COUNSELING INFORMATION
Patients must be informed that phentermine hydrochloride is a - short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Phentermine Hydrochloride Capsules USP CIV - 30 mg - Blue/Clear - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information