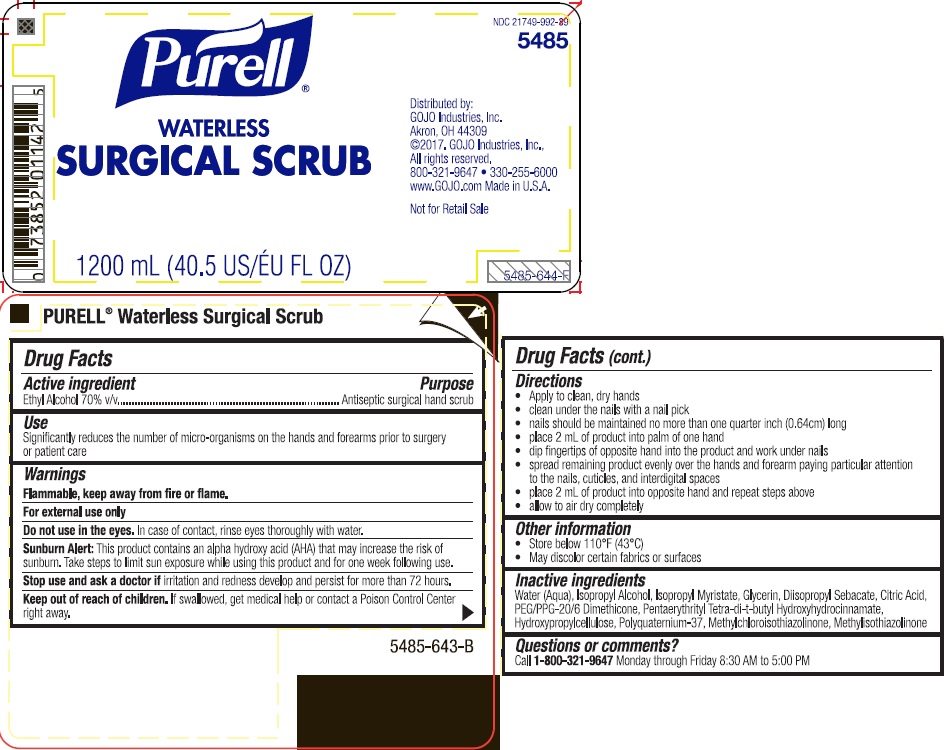
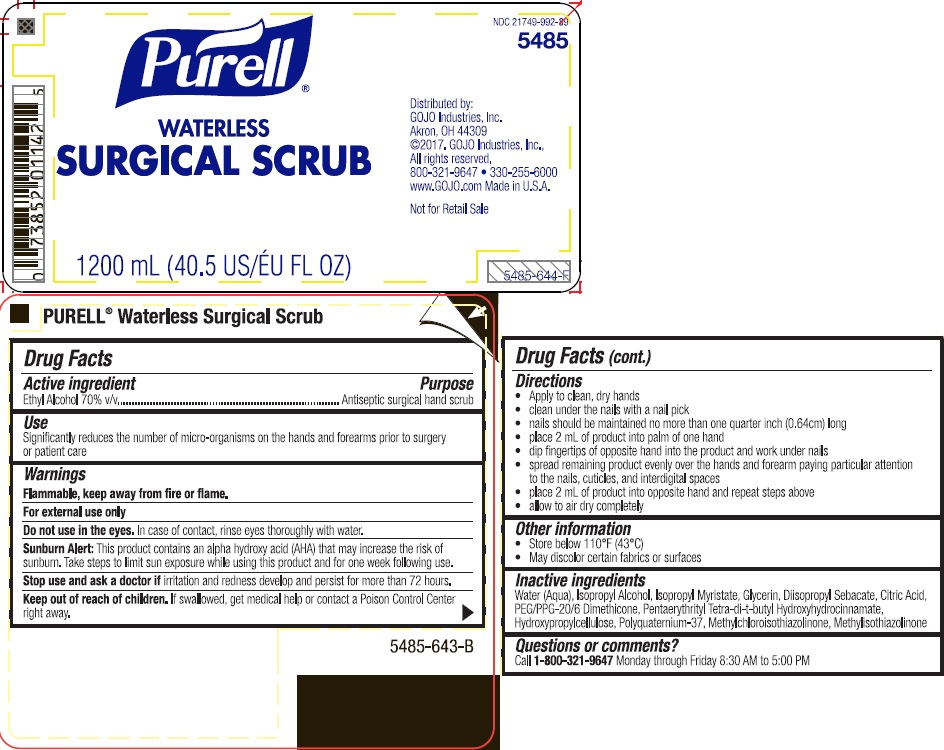
Label: PURELL WATERLESS SURGICAL SCRUB- alcohol liquid
- NDC Code(s): 21749-992-02, 21749-992-33, 21749-992-89
- Packager: GOJO Industries, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- clean under the nails with a nail pick
- mails should be maintained with a 1 millimeter free edge
- place 2 mL of product into palm of one hand
- dip fingers of opposite hand into the product and work under nails
- spread remaining product evenly over the hands and lower 2/3 of one forearm paying particular attention to the nails, cuticles, and interdigital spaces
- place 2 mL of product into opposite hand and repeat steps above
- allow to air dry completely
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PURELL WATERLESS SURGICAL SCRUB
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21749-992 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) GLYCERIN (UNII: PDC6A3C0OX) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21749-992-02 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/15/2011 2 NDC:21749-992-33 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/15/2011 03/31/2023 3 NDC:21749-992-89 1200 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/15/2011 Labeler - GOJO Industries, Inc. (004162038) Establishment Name Address ID/FEI Business Operations GOJO Industries, Inc. 036424534 manufacture(21749-992) Establishment Name Address ID/FEI Business Operations GOJO Industries, Inc. 088312414 manufacture(21749-992) , label(21749-992) , pack(21749-992)