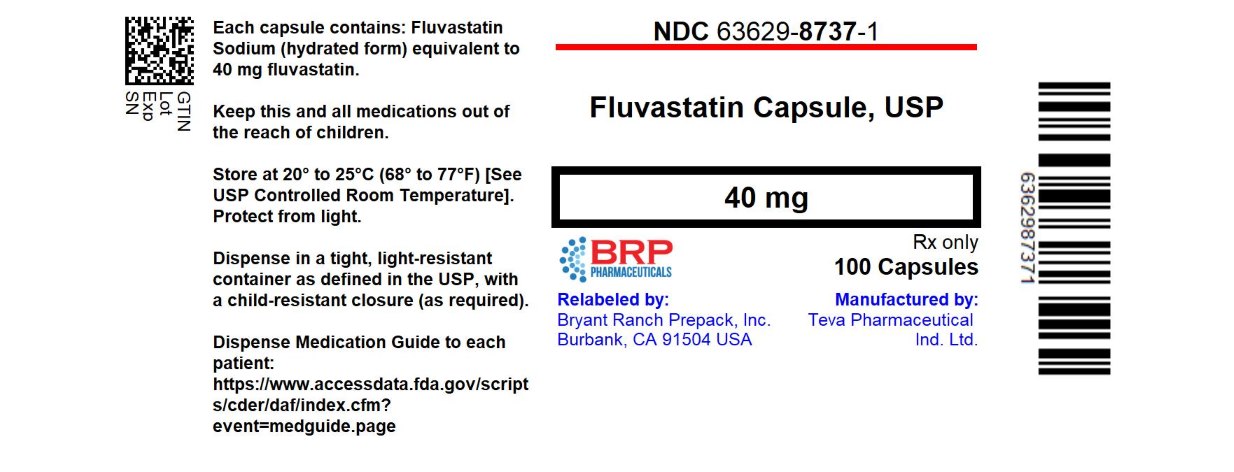
Label: FLUVASTATIN capsule
- NDC Code(s): 63629-8737-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0093-7443
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLUVASTATIN CAPSULES safely and effectively. See full prescribing information for FLUVASTATIN CAPSULES.
FLUVASTATIN capsules, for oral use
Initial U.S. Approval: 1993INDICATIONS AND USAGE
Fluvastatin capsules are an HMG-CoA reductase inhibitor (statin) indicated as an adjunctive therapy to diet to:
- Reduce elevated TC, LDL-C, Apo B, and TG, and to increase HDL-C in adult patients with primary hypercholesterolemia and mixed dyslipidemia (1.1)
- Reduce elevated TC, LDL-C, and Apo B levels in boys and post-menarchal girls, 10 to 16 years of age, with heterozygous familial hypercholesterolemia after failing an adequate trial of diet therapy (1.1)
- Reduce the risk of undergoing revascularization procedures in patients with clinically evident CHD (1.2)
- Slow the progression of atherosclerosis in patients with CHD (1.2)
Limitations of Use:
- Fluvastatin capsules have not been studied in conditions where the major abnormality is elevation of chylomicrons, VLDL, or IDL (i.e., hyperlipoproteinemia Types I, III, IV, or V). (1.3)
DOSAGE AND ADMINISTRATION
- Dose range: 20 mg to 80 mg/day (2.1)
- Fluvastatin capsules can be taken with or without food. (2.1)
- Do not open fluvastatin capsules prior to administration (2.1)
- Adults: the recommended starting dose is 40 mg to 80 mg (administered as one fluvastatin capsule, 40 mg twice daily) (2.2)
- Do not take two fluvastatin capsules, 40 mg at one time
- Children with heterozygous familial hypercholesterolemia (ages 10 to 16, inclusive): the recommended starting dose is fluvastatin capsule, 20 mg once daily (2.3)
DOSAGE FORMS AND STRENGTHS
Capsules: 20 mg, 40 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Skeletal muscle effects (e.g., myopathy and rhabdomyolysis): Risks increase with advanced age (≥ 65), uncontrolled hypothyroidism, renal impairment, and combination use with cyclosporine,or gemfibrozil. Advise patients to promptly report to their physician unexplained and/or persistent muscle pain, tenderness, or weakness and discontinue fluvastatin if myopathy is diagnosed or suspected. (5.1, 8.5, 8.7)
- Patients should be advised to report promptly any symptoms of myopathy. Fluvastatin capsules therapy should be discontinued if myopathy is diagnosed or suspected (5.1)
- Immune-Mediated Necrotizing Myopathy (IMNM): There have been rare reports of IMNM, an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents. (5.2)
- Liver enzyme abnormalities: Persistent elevations in hepatic transaminases can occur. Check liver enzyme tests before initiating therapy and as clinically indicated thereafter (5.3)
ADVERSE REACTIONS
Most frequent adverse reactions (rate ≥ 2% and > placebo) are: headache, dyspepsia, myalgia, abdominal pain and nausea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Cyclosporine: Combination increases fluvastatin exposure. Limit fluvastatin dose to 20 mg (2.4, 7.1)
- Fluconazole: Combination increases fluvastatin exposure. Limit fluvastatin dose to 20 mg (2.5, 7.2)
- Concomitant lipid-lowering therapies: Use with fibrates or lipid-modifying doses (≥ 1 g/day) of niacin increases the risk of adverse skeletal muscle effects. Caution should be used when prescribing with fluvastatin (5.1, 7.3, 7.4)
- Glyburide: Monitor blood glucose levels when fluvastatin dose is changed (7)
- Phenytoin: Monitor plasma phenytoin levels when fluvastatin treatment is initiated or when the dosage is changed (7)
- Warfarin and coumarin derivates: Monitor prothrombin times when fluvastatin coadministration is initiated, discontinued, or the dosage changed (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
1.2 Secondary Prevention of Cardiovascular Disease
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Adult Patients With Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
2.3 Pediatric Patients (10 to 16 Years of Age) With Heterozygous Familial Hypercholesterolemia
2.4 Use With Cyclosporine
2.5 Use With Fluconazole
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity to any Component of This Medication
4.2 Active Liver Disease
4.3 Pregnancy
4.4 Nursing Mothers
5 WARNINGS AND PRECAUTIONS
5.1 Skeletal Muscle
5.2 Immune-Mediated Necrotizing Myopathy
5.3 Liver Enzymes
5.4 Endocrine Effects
5.5 CNS Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience in Adult Patients
6.2 Clinical Studies Experience in Pediatric Patients
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Cyclosporine
7.2 Fluconazole
7.3 Gemfibrozil
7.4 Other Fibrates
7.5 Niacin
7.6 Glyburide
7.7 Phenytoin
7.8 Warfarin
7.9 Colchicine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
14.2 Heterozygous Familial Hypercholesterolemia in Pediatric Patients
14.3 Secondary Prevention of Cardiovascular Disease
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Muscle Pain
17.2 Liver Enzymes
17.3 Pregnancy
17.4 Breastfeeding
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Drug therapy is indicated as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other non-pharmacologic measures alone has been inadequate.
1.1 Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
Fluvastatin capsules are indicated
- as an adjunct to diet to reduce elevated total cholesterol (Total-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG) and apolipoprotein B (Apo B) levels, and to increase high-density lipoprotein cholesterol (HDL-C) in patients with primary hypercholesterolemia and mixed dyslipidemia (Fredrickson Type IIa and IIb).
- as an adjunct to diet to reduce Total-C, LDL-C, and Apo B levels in adolescent boys and adolescent girls who are at least one year post-menarche, 10 to 16 years of age, with heterozygous familial hypercholesterolemia and the following findings are present:
- LDL-C remains ≥ 190 mg/dL or
- LDL-C remains ≥ 160 mg/dL and:
- there is a positive family history of premature cardiovascular disease or
- two or more other cardiovascular disease risk factors are present
The NCEP classification of cholesterol levels in pediatric patients with a familial history of hypercholesterolemia or premature CVD is summarized below.
Category
Total-C (mg/dL)
LDL-C (mg/dL)
Acceptable
< 170
< 110
Borderline
170 to 199
110 to 129
High
≥ 200
≥ 130
Children treated with fluvastatin in adolescence should be re-evaluated in adulthood and appropriate changes made to their cholesterol-lowering regimen to achieve adult treatment goals.
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
Dose range: 20 mg to 80 mg/day.
Fluvastatin capsules can be administered orally as a single dose, with or without food.
Do not open fluvastatin capsules prior to administration.
Do not take two fluvastatin capsules, 40 mg at one time.
Since the maximal effect of a given dose is seen within 4 weeks, periodic lipid determinations should be performed at this time and dosage adjusted according to the patient’s response to therapy and established treatment guidelines.
For patients requiring LDL-C reduction to a goal of ≥ 25%, the recommended starting dose is 40 mg as one capsule in the evening, or 80 mg in divided doses of the 40 mg capsule given twice daily. For patients requiring LDL-C reduction to a goal of < 25% a starting dose of 20 mg may be used.
2.2 Adult Patients With Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
Adult patients can be started on fluvastatin capsules. The recommended starting dose for fluvastatin capsules is one 40 mg capsule in the evening, or one fluvastatin capsule, 40 mg twice daily. Do not take two fluvastatin capsules, 40 mg at one time.
2.3 Pediatric Patients (10 to 16 Years of Age) With Heterozygous Familial Hypercholesterolemia
The recommended starting dose is one fluvastatin capsule, 20 mg. Dose adjustments, up to a maximum daily dose administered as fluvastatin capsules, 40 mg twice daily should be made at 6 week intervals. Doses should be individualized according to the goal of therapy [see NCEP Pediatric Panel Guidelines and CLINICAL STUDIES (14)]1.
1 National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 89(3):495-501. 1992.
2.4 Use With Cyclosporine
Do not exceed a dose of 20 mg b.i.d. fluvastatin capsules in patients taking cyclosporine [see Drug Interactions (7.1)].
2.5 Use With Fluconazole
Do not exceed a dose of 20 mg b.i.d. fluvastatin capsules in patients taking fluconazole [see Drug Interactions (7.2)].
-
3 DOSAGE FORMS AND STRENGTHS
- 20 mg are hard gelatin capsules with ivory opaque body and pink opaque cap, filled with an off-white to yellowish powder with small agglomerates. Cap imprinted with “TEVA” and body imprinted with “7442”.
- 40 mg are hard gelatin capsules with yellow opaque body and pink opaque cap, filled with an off-white to yellowish powder with small agglomerates. Cap imprinted with “TEVA” and body imprinted with “7443”.
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity to any Component of This Medication
Fluvastatin capsules are contraindicated in patients with hypersensitivity to any component of this medication.
4.2 Active Liver Disease
Fluvastatin capsules are contraindicated in patients with active liver disease or unexplained, persistent elevations in serum transaminases [see Warnings and Precautions (5.3)].
4.3 Pregnancy
Fluvastatin capsules are contraindicated in women who are pregnant or may become pregnant. Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol or cholesterol derivatives are essential for fetal development. Fluvastatin capsules may cause fetal harm when administered to pregnant women. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia.
Fluvastatin capsules should be administered to women of childbearing age only when such patients are highly unlikely to conceive and have been informed of the potential hazards. If the patient becomes pregnant while taking this drug, fluvastatin capsules should be discontinued and the patient should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
4.4 Nursing Mothers
Fluvastatin is secreted into the breast milk of animals and because HMG-CoA reductase inhibitors have the potential to cause serious adverse reactions in nursing infants, women who require treatment with fluvastatin capsules should be advised not to breastfeed their infants [see Use in Specific Populations (8.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Skeletal Muscle
Rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with fluvastatin capsules and other drugs in this class.
Fluvastatin capsules should be prescribed with caution in patients with predisposing factors for myopathy. These factors include advanced age (> 65 years), renal impairment, and inadequately treated hypothyroidism.
The risk of myopathy and/or rhabdomyolysis with statins is increased with concurrent therapy with cyclosporine, erythromycin, fibrates or niacin. Myopathy was not observed in a clinical trial in 74 patients involving patients who were treated with fluvastatin sodium together with niacin. Isolated cases of myopathy have been reported during postmarketing experience with concomitant administration of fluvastatin sodium and colchicine. No information is available on the pharmacokinetic interaction between fluvastatin sodium and colchicine.
Uncomplicated myalgia has also been reported in fluvastatin sodium-treated patients [see Adverse Reactions (6)]. In clinical trials, uncomplicated myalgia has been observed infrequently in patients treated with fluvastatin sodium at rates indistinguishable from placebo. Myopathy, defined as muscle aching or muscle weakness in conjunction with increases in CPK values to greater than 10 times the upper limit of normal, was < 0.1% in fluvastatin clinical trials. Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevation of CPK.
All patients should be advised to promptly report to their physician unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing fluvastatin.
Fluvastatin sodium therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Fluvastatin sodium therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
5.2 Immune-Mediated Necrotizing Myopathy
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents. Additional neuromuscular and serologic testing may be necessary. Treatment with immunosuppressive agents may be required. Consider risk of IMNM carefully prior to initiation of a different statin. If therapy is initiated with a different statin, monitor for signs and symptoms of IMNM.
5.3 Liver Enzymes
Increases in serum transaminases (aspartate aminotransferase [AST]/serum glutamic-oxaloacetic transaminase, or alanine aminotransferase [ALT]/serum glutamic-pyruvic transaminase) have been reported with HMG-CoA reductase inhibitors, including fluvastatin sodium. In most cases, the elevations were transient and resolved or improved on continued therapy or after a brief interruption in therapy.
Approximately 1.1% of patients treated with fluvastatin capsules in worldwide trials developed dose-related, persistent elevations of serum transaminase levels to more than 3 times the upper limit of normal. Fourteen of these patients (0.6%) were discontinued from therapy. In all clinical trials, a total of 33/2969 patients (1.1%) had persistent transaminase elevations with an average fluvastatin sodium exposure of approximately 71.2 weeks; 19 of these patients (0.6%) were discontinued. The majority of patients with these abnormal biochemical findings were asymptomatic.
In a pooled analysis of all placebo-controlled studies in which fluvastatin capsules were used, persistent transaminase elevations (> 3 times the upper limit of normal [ULN] on two consecutive weekly measurements) occurred in 0.2%, 1.5%, and 2.7% of patients treated with daily doses of 20, 40, and 80 mg (titrated to 40 mg twice daily) fluvastatin capsules, respectively. Ninety-one percent of the cases of persistent liver function test abnormalities (20 of 22 patients) occurred within 12 weeks of therapy and in all patients with persistent liver function test abnormalities there was an abnormal liver function test present at baseline or by Week 8.
In the pooled analysis of the 24 week controlled trials, persistent transaminase elevation occurred in 1.8% and 4.9% of patients treated with fluvastatin capsules, 40 mg and fluvastatin capsules, 40 mg twice daily, respectively.
It is recommended that liver enzyme tests be performed prior to the initiation of fluvastatin sodium, and if signs or symptoms of liver injury occur.
There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including fluvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with fluvastatin sodium, promptly interrupt therapy. If an alternate etiology is not found do not restart fluvastatin sodium.
In very rare cases, possibly drug-related hepatitis was observed that resolved upon discontinuation of treatment.1 Active liver disease or unexplained serum transaminase elevations are contraindications to the use of fluvastatin sodium [see Contraindications (4) and Warnings and Precautions (5.3)]. Caution should be exercised when fluvastatin sodium is administered to patients with a history of liver disease or heavy alcohol ingestion [see Clinical Pharmacology (12.3)]. Such patients should be closely monitored.
5.4 Endocrine Effects
Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including fluvastatin sodium.
Statins interfere with cholesterol synthesis and lower circulating cholesterol levels and, as such, might theoretically blunt adrenal or gonadal steroid hormone production.
Fluvastatin sodium exhibited no effect upon non-stimulated cortisol levels and demonstrated no effect upon thyroid metabolism as assessed by measurement of thyroid stimulating hormone (TSH). Small declines in total serum testosterone have been noted in treated groups, but no commensurate elevation in LH occurred, suggesting that the observation was not due to a direct effect upon testosterone production. No effect upon FSH in males was noted. Due to the limited number of premenopausal females studied to date, no conclusions regarding the effect of fluvastatin sodium upon female sex hormones may be made.
Two clinical studies in patients receiving fluvastatin at doses up to 80 mg daily for periods of 24 to 28 weeks demonstrated no effect of treatment upon the adrenal response to ACTH stimulation. A clinical study evaluated the effect of fluvastatin at doses up to 80 mg daily for 28 weeks upon the gonadal response to HCG stimulation. Although the mean total testosterone response was significantly reduced (p < 0.05) relative to baseline in the 80 mg group, it was not significant in comparison to the changes noted in groups receiving either 40 mg of fluvastatin or placebo.
Patients treated with fluvastatin sodium who develop clinical evidence of endocrine dysfunction should be evaluated appropriately. Caution should be exercised if a statin or other agent used to lower cholesterol levels is administered to patients receiving other drugs (e.g., ketoconazole, spironolactone, cimetidine) that may decrease the levels of endogenous steroid hormones.
5.5 CNS Toxicity
CNS effects, as evidenced by decreased activity, ataxia, loss of righting reflex, and ptosis were seen in the following animal studies: the 18 month mouse carcinogenicity study at 50 mg/kg/day, the 6 month dog study at 36 mg/kg/day, the 6 month hamster study at 40 mg/kg/day, and in acute, high-dose studies in rats and hamsters (50 mg/kg), rabbits (300 mg/kg) and mice (1500 mg/kg). CNS toxicity in the acute high-dose studies was characterized (in mice) by conspicuous vacuolation in the ventral white columns of the spinal cord at a dose of 5000 mg/kg and (in rats) by edema with separation of myelinated fibers of the ventral spinal tracts and sciatic nerve at a dose of 1500 mg/kg. CNS toxicity, characterized by periaxonal vacuolation, was observed in the medulla of dogs that died after treatment for 5 weeks with 48 mg/kg/day; this finding was not observed in the remaining dogs when the dose level was lowered to 36 mg/kg/day. CNS vascular lesions, characterized by perivascular hemorrhages, edema, and mononuclear cell infiltration of perivascular spaces, have been observed in dogs treated with other members of this drug class. No CNS lesions have been observed after chronic treatment for up to 2 years with fluvastatin in the mouse (at doses up to 350 mg/kg/day), rat (up to 24 mg/kg/day), or dog (up to 16 mg/kg/day).
Prominent bilateral posterior Y suture lines in the ocular lens were seen in dogs after treatment with 1, 8, and 16 mg/kg/day for 2 years.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Rhabdomyolysis with myoglobinuria and acute renal failure and myopathy (including myositis) [see Warnings and Precautions (5.1)].
- Liver Enzyme Abnormalities [see Warnings and Precautions (5.3)].
6.1 Clinical Studies Experience in Adult Patients
Because clinical studies on fluvastatin capsules are conducted in varying study populations and study designs, the frequency of adverse reactions observed in the clinical studies of fluvastatin capsules cannot be directly compared with that in the clinical studies of other statins and may not reflect the frequency of adverse reactions observed in clinical practice.
In the fluvastatin capsules placebo-controlled clinical trials database of 2326 patients treated with fluvastatin capsules1 (age range 18 to 75 years, 44% women, 94% Caucasians, 4% Blacks, 2% other ethnicities) with a median treatment duration of 24 weeks, 3.4% of patients on fluvastatin capsules and 2.3% patients on placebo discontinued due to adverse reactions regardless of causality. The most common adverse reactions that led to treatment discontinuation and occurred at an incidence greater than placebo were: transaminase increased (0.8%), upper abdominal pain (0.3%), dyspepsia (0.3%), fatigue (0.2%) and diarrhea (0.2%).
Clinically relevant adverse experiences occurring in the fluvastatin capsules controlled studies with a frequency ≥ 2%, regardless of causality, included the following:
Table 1: Clinical Adverse Events Reported in > 2% in Patients Treated With Fluvastatin Capsules and at an Incidence Greater Than Placebo in Placebo-Controlled Trials Regardless of Causality (% of Patients) Pooled Dosages Fluvastatin Capsules1
N = 2326
(%)
Placebo1
N = 960
(%)
Musculoskeletal
Myalgia
5.0
4.5
Arthritis
2.1
2.0
Arthropathy
NA
NA
Respiratory
Sinusitis
2.6
1.9
Bronchitis
1.8
1.0
Gastrointestinal
Dyspepsia
7.9
3.2
Diarrhea
4.9
4.2
Abdominal pain
4.9
3.8
Nausea
3.2
2.0
Flatulence
2.6
2.5
Tooth disorder
2.1
1.7
Psychiatric
Insomnia
2.7
1.4
Genitourinary
Urinary tract infection
1.6
1.1
Miscellaneous
Headache
8.9
7.8
Influenza-like symptoms
5.1
5.7
Accidental Trauma
5.1
4.8
Fatigue
2.7
2.3
Allergy
2.3
2.2
1. Controlled trials with fluvastatin capsules (20 and 40 mg daily and 40 mg twice daily) compared to placebo
Fluvastatin Capsules Intervention Prevention Study
In the Fluvastatin Capsules Intervention Prevention Study, the effect of fluvastatin capsules, 40 mg, administered twice daily on the risk of recurrent cardiac events was assessed in 1677 patients with CHD who had undergone a percutaneous coronary intervention (PCI) procedure. This was a multicenter, randomized, double-blind, placebo-controlled study, patients were treated with dietary/lifestyle counseling and either fluvastatin capsules, 40 mg (n = 844) or placebo (n = 833) given twice daily for a median of 3.9 years [see Clinical Studies (14.3)].
Table 2: Clinical Adverse Events Reported in ≥ 2% in Patients Treated With Fluvastatin Sodium and at an Incidence Greater Than Placebo in the Trial Regardless of Causality (% of Patients) Fluvastatin Capsules,
40 mg b.i.d.
N = 822
(%)
Placebo
N = 818
(%)
Cardiac disorders
Atrial fibrillation
2.4
2.0
Gastrointestinal disorders
Abdominal pain upper
6.3
4.5
Constipation
3.3
2.1
Dyspepsia
4.5
4.0
Gastric disorder
2.7
2.1
Nausea
2.7
2.3
General disorders
Fatigue
4.7
3.8
Edema peripheral
4.4
2.9
Infections and infestations
Bronchitis
2.3
2.0
Nasopharyngitis
2.8
2.1
Musculoskeletal and connective tissue disorders
Arthralgia
2.1
1.8
Myalgia
2.2
1.6
Pain in extremity
4.1
2.7
Nervous system disorders
Dizziness
3.9
3.5
Syncope
2.4
2.2
Respiratory disorders
Dyspnea exertional
2.8
2.4
Vascular disorders
Hypertension
5.8
4.2
Intermittent claudication
2.3
2.1
6.2 Clinical Studies Experience in Pediatric Patients
In patients aged < 18 years, efficacy and safety have not been studied for treatment periods longer than two years.
In two open-label, uncontrolled studies, 66 boys and 48 girls with heterozygous familial hypercholesterolemia (9 to 16 years of age, 80% Caucasian, 19% Other [mixed ethnicity], 1% Asians) were treated with fluvastatin sodium administered as fluvastatin capsules, 20 mg to 40 mg twice daily, or fluvastatin sodium extended-release tablets, 80 mg [see Clinical Studies (14.2) and Use in Specific Populations (8.4)].
6.3 Postmarketing Experience
Because adverse reactions from spontaneous reports are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following effects have been reported with drugs in this class. Not all the effects listed below have necessarily been associated with fluvastatin sodium therapy.
Musculoskeletal: muscle cramps, myalgia, myopathy, rhabdomyolysis, arthralgias, muscle spasms, muscle weakness, myositis.
There have been rare reports of immune-mediated necrotizing myopathy associated with statin use [see Warnings and Precautions (5.1)].
Neurological: dysfunction of certain cranial nerves (including alteration of taste, impairment of extra-ocular movement, facial paresis), tremor, dizziness, vertigo, paresthesia, hypoesthesia, dysesthesia, peripheral neuropathy, peripheral nerve palsy.
There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
Psychiatric: anxiety, insomnia, depression, psychic disturbances
Respiratory: interstitial lung disease
Hypersensitivity Reactions: An apparent hypersensitivity syndrome has been reported rarely which has included one or more of the following features: anaphylaxis, angioedema, lupus erythematosus-like syndrome, polymyalgia rheumatica, vasculitis, purpura, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR (erythrocyte sedimentation rate) increase, eosinophilia, arthritis, arthralgia, urticaria, asthenia, photosensitivity reaction, fever, chills, flushing, malaise, dyspnea, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
Gastrointestinal: pancreatitis, hepatitis, including chronic active hepatitis, cholestatic jaundice, fatty change in liver, cirrhosis, fulminant hepatic necrosis, hepatoma, anorexia, vomiting, fatal and non-fatal hepatic failure.
Skin: rash, dermatitis, including bullous dermatitis, eczema, alopecia, pruritus, a variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails).
Reproductive: gynecomastia, loss of libido, erectile dysfunction.
Eye: progression of cataracts (lens opacities), ophthalmoplegia.
Laboratory abnormalities: elevated transaminases, alkaline phosphatase, gamma-glutamyl transpeptidase and bilirubin; thyroid function abnormalities.
-
7 DRUG INTERACTIONS
7.1 Cyclosporine
Cyclosporine coadministration increases fluvastatin exposure. Therefore, in patients taking cyclosporine, therapy should be limited to fluvastatin 20 mg twice daily [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 Fluconazole
Administration of fluvastatin 40 mg single dose to healthy volunteers pre-treated with fluconazole for 4 days results in an increase of fluvastatin exposure. Therefore, in patients taking fluconazole, therapy should be limited to fluvastatin 20 mg twice daily [see Clinical Pharmacology (12.3)].
7.3 Gemfibrozil
Due to an increased risk of myopathy/rhabdomyolysis when HMG-CoA reductase inhibitors are coadministered with gemfibrozil, concomitant administration of fluvastatin sodium with gemfibrozil should be avoided.
7.4 Other Fibrates
Because it is known that the risk of myopathy during treatment with HMG-CoA reductase inhibitors is increased with concurrent administration of other fibrates, fluvastatin sodium should be administered with caution when used concomitantly with other fibrates [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.5 Niacin
The risk of skeletal muscle effects may be enhanced when fluvastatin sodium is used in combination with lipid-modifying doses (≥ 1 g/day) of niacin; a reduction in fluvastatin sodium dosage should be considered in this setting [see Warnings and Precautions (5.1)].
7.6 Glyburide
Concomitant administration of fluvastatin and glyburide increased glyburide exposures. Patients on concomitant therapy of glyburide and fluvastatin should continue to be monitored appropriately [see Clinical Pharmacology (12.3)].
7.7 Phenytoin
Concomitant administration of fluvastatin and phenytoin increased phenytoin exposures. Patients should continue to be monitored appropriately when fluvastatin therapy is initiated or when fluvastatin dose is changed [see Clinical Pharmacology (12.3)].
7.8 Warfarin
Bleeding and/or increased prothrombin times have been reported in patients taking coumarin anticoagulants concomitantly with other HMG-CoA reductase inhibitors. Therefore, patients receiving warfarin-type anticoagulants should have their prothrombin times closely monitored when fluvastatin sodium is initiated or the dosage of fluvastatin sodium is changed.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X
Fluvastatin sodium is contraindicated in women who are or may become pregnant [see Contraindications (4)].
Lipid lowering drugs are contraindicated during pregnancy, because cholesterol and cholesterol derivatives are needed for normal fetal development. Serum cholesterol and triglycerides increase during normal pregnancy. Atherosclerosis is a chronic process, and discontinuation of lipid-lowering drugs during pregnancy should have little impact on long-term outcomes of primary hypercholesterolemia therapy.
There are no adequate and well-controlled studies of use with fluvastatin sodium during pregnancy. Rare reports of congenital anomalies have been received following intrauterine exposure to other statins. In a review2 of about 100 prospectively followed pregnancies in women exposed to other statins, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed the rate expected in the general population. The number of cases is adequate only to exclude a 3 to 4 fold increase in congenital anomalies over background incidence. In 89% of prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified.
Teratology studies with fluvastatin in rats and rabbits showed maternal toxicity at high dose levels, but there was no evidence of embryotoxic or teratogenic potential [see Nonclinical Toxicology (13)].
Fluvastatin sodium should be administered to women of child-bearing potential only when such patients are highly unlikely to conceive and have been informed of the potential hazards. If a woman becomes pregnant while taking fluvastatin sodium, the drug should be discontinued and the patient advised again as to the potential hazards to the fetus.
8.3 Nursing Mothers
Based on animal data, fluvastatin is present in breast milk in a 2:1 ratio (milk:plasma). Because of the potential for serious adverse reactions in nursing infants, nursing women should not take fluvastatin sodium [see Contraindications (4)].
8.4 Pediatric Use
The safety and efficacy of fluvastatin sodium in children and adolescent patients 9 to 16 years of age with heterozygous familial hypercholesterolemia have been evaluated in open-label, uncontrolled clinical trials for a duration of two years. The most common adverse events observed were influenza and infections. In these limited uncontrolled studies, there was no detectable effect on growth or sexual maturation in the adolescent boys or on menstrual cycle length in girls [see Clinical Studies (14.2), Adverse Reactions (6.3), and Dosage and Administration (2.2)]. Adolescent females should be counseled on appropriate contraceptive methods while on fluvastatin sodium therapy [see Contraindications (4)].
8.5 Geriatric Use
Fluvastatin exposures were not significantly different between the nonelderly and elderly populations (age ≥ 65 years) [see Clinical Pharmacology (12.3)]. Since advanced age (≥ 65 years) is a predisposing factor for myopathy, fluvastatin sodium should be prescribed with caution in the elderly.
8.6 Hepatic Impairment
Fluvastatin sodium is contraindicated in patients with active liver disease or unexplained, persistent elevations in serum transaminases [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Dose adjustments for mild to moderate renal impairment are not necessary. Fluvastatin has not been studied at doses greater than 40 mg in patients with severe renal impairment; therefore caution should be exercised when treating such patients at higher doses [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
To date, there has been limited experience with overdosage of fluvastatin. If an overdose occurs, it should be treated symptomatically with laboratory monitoring and supportive measures should be instituted as required. The dialyzability of fluvastatin sodium and of its metabolites in humans is not known at present [see Warnings and Precautions (5)].
In the pediatric population, there have been reports of overdosage with fluvastatin sodium in children including a 2-year-old and the other 3 years of age, either of whom may have possibly ingested fluvastatin sodium. The maximum amount of fluvastatin sodium that could have been ingested was 80 mg (4 x 20 mg capsules). Vomiting was induced by ipecac in both children and no capsules were noted in their emesis. Neither child experienced any adverse symptoms and both recovered from the incident without problems.
In the postmarketing experience there have been reports of accidental ingestion of fluvastatin tablets in infants up to 3 years of age. In one case, increased serum CPK values were noted. There have been reports of intentional overdose in adolescents with the development of hepatic enzyme elevations, convulsions and gastroenteritis/vomiting/diarrhea.
-
11 DESCRIPTION
Fluvastatin sodium, USP is a water-soluble cholesterol lowering agent which acts through the inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase.
Fluvastatin sodium, USP is [R*,S*-(E)]-(±)-7-[3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid, monosodium salt. Its structural formula is:
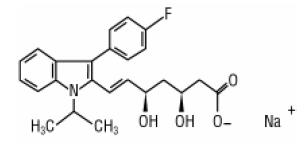
C24H25FNNaO4 M.W. 433.45
This molecular entity is the first entirely synthetic HMG-CoA reductase inhibitor, and is in part structurally distinct from the fungal derivatives of this therapeutic class.
Fluvastatin sodium, USP (hydrated form) is a white to pale yellow, brownish-pale yellow, or reddish-pale yellow, hygroscopic powder soluble in water, ethanol, and methanol. Fluvastatin Capsules, USP contain fluvastatin sodium, USP (hydrated form), equivalent to 20 mg or 40 mg of fluvastatin, for oral administration.
Active Ingredient: fluvastatin sodium, USP (hydrated form)
Inactive Ingredients: black iron oxide, colloidal silicon dioxide, crospovidone, gelatin, lactose monohydrate, magnesium stearate, propylene glycol, red iron oxide, shellac, titanium dioxide, and yellow iron oxide. The imprinting ink may contain potassium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fluvastatin sodium is a competitive inhibitor of HMG-CoA reductase, the rate limiting enzyme that converts 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) to mevalonate, a precursor of sterols, including cholesterol. The inhibition of cholesterol biosynthesis reduces the cholesterol in hepatic cells, which stimulates the synthesis of LDL receptors and thereby increases the uptake of LDL particles. The end result of these biochemical processes is a reduction of the plasma cholesterol concentration.
12.3 Pharmacokinetics
Absorption:
Following oral administration of the capsule, fluvastatin reaches peak concentrations in less than 1 hour. The absolute bioavailability is 24% (range 9% to 50%) after administration of a 10 mg dose.
At steady state, administration of fluvastatin with the evening meal results in a 50% decrease in Cmax, an 11% decrease in AUC, and a more than two-fold increase in tmax as compared to administration 4 hours after the evening meal. No significant differences in the lipid-lowering effects were observed between the two administrations. After single or multiple doses above 20 mg, fluvastatin exhibits saturable first-pass metabolism resulting in more than dose proportional plasma fluvastatin concentrations.
Fluvastatin administered as fluvastatin sodium extended-release 80 mg tablets reaches peak concentration in approximately 3 hours under fasting conditions, after a low fat meal, or 2.5 hours after a low fat meal. The mean relative bioavailability of the extended-release tablet is approximately 29% (range: 9% to 66%) compared to that of the fluvastatin immediate-release capsule administered under fasting conditions. Administration of a high fat meal delayed the absorption (Tmax: 6h) and increased the bioavailability of the extended-release tablet by approximately 50%. However, the maximum concentration of fluvastatin sodium extended-release tablets seen after a high fat meal is less than the peak concentration following a single dose or twice daily dose of the 40 mg fluvastatin capsule.
Distribution:
Fluvastatin is 98% bound to plasma proteins. The mean volume of distribution (VDss) is estimated at 0.35 L/kg. At therapeutic concentrations, the protein binding of fluvastatin is not affected by warfarin, salicylic acid and glyburide.
Metabolism:
Fluvastatin is metabolized in the liver, primarily via hydroxylation of the indole ring at the 5 and 6 positions. N-dealkylation and beta-oxidation of the side-chain also occurs. The hydroxy metabolites have some pharmacologic activity, but do not circulate in the blood. Fluvastatin has two enantiomers. Both enantiomers of fluvastatin are metabolized in a similar manner.
In vitro data indicate that fluvastatin metabolism involves multiple Cytochrome P450 (CYP) isozymes. CYP2C9 isoenzyme is primarily involved in the metabolism of fluvastatin (approximately 75%), while CYP2C8 and CYP3A4 isoenzymes are involved to a much less extent, i.e., approximately 5% and approximately 20%, respectively.
Excretion:
Following oral administration, fluvastatin is primarily (about 90%) excreted in the feces as metabolites, with less than 2% present as unchanged drug. Approximately 5% of a radiolabeled oral dose were recovered in urine. The elimination half-life (t1/2) of fluvastatin is approximately 3 hours.
Specific Populations
Renal Impairment:
In patients with moderate to severe renal impairment (CLCr 10 to 40 mL/min), AUC and Cmax increased approximately 1.2 fold after administration of a single dose of 40 mg fluvastatin compared to healthy volunteers. In patients with end-stage renal disease on hemodialysis, the AUC increased by approximately 1.5 fold. Fluvastatin sodium extended-release tablets were not evaluated in patients with renal impairment. However, systemic exposures after administration of fluvastatin sodium extended-release tablets are lower than after the 40 mg immediate-release capsule.
Hepatic Impairment:
In patients with hepatic impairment due to liver cirrhosis, fluvastatin AUC and Cmax increased approximately 2.5 fold compared to healthy subjects after administration of a single 40 mg dose. The enantiomer ratios of the two isomers of fluvastatin in hepatic impairment patients were comparable to those observed in healthy subjects.
Geriatric:
Plasma levels of fluvastatin are not significantly different in patients age > 65 years compared to patients age 21 to 49 years.
Gender:
In a study evaluating the effect of age and gender on fluvastatin pharmacokinetics, there were no significant differences in fluvastatin exposures between males and females, except between younger females and younger males (both ages 21 to 49 years), where there was an approximate 30% increase in AUC in females. Adjusting for body weight decreases the magnitude of the differences seen.
Pediatric:
Pharmacokinetic data in the pediatric population are not available.
Drug-Drug Interactions:
Data from drug-drug interactions studies involving coadministration of gemfibrozil, niacin, itraconazole, erythromycin, tolbutamide or clopidogrel indicate that the PK disposition of fluvastatin is not significantly altered when fluvastatin is coadministered with any of these drugs.
The below listed drug interaction information is derived from studies using fluvastatin capsules.
Table 3: Effect of Coadministered Drugs on Fluvastatin Systemic Exposure Coadministered drug and dosing regimen
Fluvastatin
Dose (mg)1
Change in AUC2
Change in Cmax2
Cyclosporine - stable dose (b.i.d.)3
20 mg QD for 14 weeks
↑90%
↑30%
Fluconazole 400 mg QD day 1,200 mg b.i.d. day 2 to 43
40 mg QD
↑84%
↑44%
Cholestyramine 8 g QD
20 mg QD administered 4 hrs after a meal plus cholestyramine
↓51%
↓83%
Rifampicin 600 mg QD for 6 days
20 mg QD
↓53%
↓42%
Cimetidine 400 mg b.i.d. for 5 days, QD on Day 6
20 mg QD
↑30%
↑40%
Ranitidine 150 mg b.i.d. for 5 days, QD on Day 6
20 mg QD
↑10%
↑50%
Omeprazole 40 mg QD for 6 days
20 mg QD
↑20%
↑37%
Phenytoin 300 mg QD
40 mg b.i.d. for 5 days
↑40%
↑27%
Propranolol 40 mg b.i.d. for 3.5 days
40 mg QD
↓5%
No change
Digoxin 0.1 to 0.5 mg QD for 3 weeks
40 mg QD
No change
↑11%
Diclofenac 25 mg QD
40 mg QD for 8 days
↑50%
↑80%
Glyburide 5 to 20 mg QD for 22 days
40 mg b.i.d. for 14 days
↑51%
↑44%
Warfarin 30 mg QD
40 mg QD for 8 days
↑30%
↑67%
Clopidogrel 300 mg loading dose on day 10, 75 mg QD on days 11 to 19
fluvastatin sodium extended-release tablets, 80 mg QD for 19 days
↓2%
↑27%
1. Single dose unless otherwise noted
2. Mean ratio (with/without coadministered drug and no change = 1 fold) or % change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively.
3. Considered clinically significant [see Dosage and Administration (2) and Drug Interactions (7)]Data from drug-drug interaction studies involving fluvastatin and coadministration of either gemfibrozil, tolbutamide or losartan indicate that the PK disposition of either gemfibrozil, tolbutamide or losartan is not significantly altered when coadministered with fluvastatin.
Table 4: Effect of Fluvastatin Coadministration on Systemic Exposure of Other Drugs Fluvastatin dosage regimen
Coadministered drug
Name and Dose (mg)1
Change in AUC2
Change in Cmax2
40 mg QD for 5 days
Phenytoin 300 mg QD3
↑20%
↑5%
40 mg b.i.d. for 21 days
Glyburide 5 to 20 mg QD for 22 days3
↑70%
↑50%
40 mg QD for 8 days
Diclofenac 25 mg QD
↑25%
↑60%
40 mg QD for 8 days
Warfarin 30 mg QD
S-warfarin: ↑7%
S-warfarin: ↑10%
R-warfarin: no change
R-warfarin: ↑6%
1. Single dose unless otherwise noted
2. Mean ratio (with/without coadministered drug and no change = 1 fold) or % change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively.
3. Considered clinically significant [see Dosage and Administration (2) and Drug Interactions (7)] -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2 year study was performed in rats at dose levels of 6, 9, and 18 to 24 (escalated after 1 year) mg/kg/day. These treatment levels represented plasma drug levels of approximately 9, 13, and 26 to 35 times the mean human plasma drug concentration after a 40 mg oral dose. A low incidence of forestomach squamous papillomas and 1 carcinoma of the forestomach at the 24 mg/kg/day dose level was considered to reflect the prolonged hyperplasia induced by direct contact exposure to fluvastatin sodium rather than to a systemic effect of the drug. In addition, an increased incidence of thyroid follicular cell adenomas and carcinomas was recorded for males treated with 18 to 24 mg/kg/day. The increased incidence of thyroid follicular cell neoplasm in male rats with fluvastatin sodium appears to be consistent with findings from other HMG-CoA reductase inhibitors. In contrast to other HMG-CoA reductase inhibitors, no hepatic adenomas or carcinomas were observed.
The carcinogenicity study conducted in mice at dose levels of 0.3, 15 and 30 mg/kg/day revealed, as in rats, a statistically significant increase in forestomach squamous cell papillomas in males and females at 30 mg/kg/day and in females at 15 mg/kg/day. These treatment levels represented plasma drug levels of approximately 0.05, 2, and 7 times the mean human plasma drug concentration after a 40 mg oral dose.
No evidence of mutagenicity was observed in vitro, with or without rat-liver metabolic activation, in the following studies: microbial mutagen tests using mutant strains of Salmonella typhimurium or Escherichia coli; malignant transformation assay in BALB/3T3 cells; unscheduled DNA synthesis in rat primary hepatocytes; chromosomal aberrations in V79 Chinese Hamster cells; HGPRT V79 Chinese Hamster cells. In addition, there was no evidence of mutagenicity in vivo in either a rat or mouse micronucleus test.
In a study in rats at dose levels for females of 0.6, 2, and 6 mg/kg/day and at dose levels for males of 2, 10 and 20 mg/kg/day, fluvastatin sodium had no adverse effects on the fertility or reproductive performance.
Seminal vesicles and testes were small in hamsters treated for 3 months at 20 mg/kg/day (approximately three times the 40 mg human daily dose based on surface area, mg/m2). There was tubular degeneration and aspermatogenesis in testes as well as vesiculitis of seminal vesicles. Vesiculitis of seminal vesicles and edema of the testes were also seen in rats treated for 2 years at 18 mg/kg/day (approximately 4 times the human Cmax achieved with a 40 mg daily dose).
Fluvastatin sodium produced delays in skeletal development in rats at doses of 12 mg/kg/day and in rabbits at doses of 10 mg/kg/day. Malaligned thoracic vertebrae were seen in rats at 36 mg/kg, a dose that produced maternal toxicity. These doses resulted in 2 times (rat at 12 mg/kg) or 5 times (rabbit at 10 mg/kg) the 40 mg human exposure based on mg/m2 surface area. A study in which female rats were dosed during the third trimester at 12 and 24 mg/kg/day resulted in maternal mortality at or near term and postpartum. In addition, fetal and neonatal lethality were apparent. No effects on the dam or fetus occurred at 2 mg/kg/day. A second study at levels of 2, 6, 12 and 24 mg/kg/day confirmed the findings in the first study with neonatal mortality beginning at 6 mg/kg. A modified Segment III study was performed at dose levels of 12 or 24 mg/kg/day with or without the presence of concurrent supplementation with mevalonic acid, a product of HMG-CoA reductase which is essential for cholesterol biosynthesis. The concurrent administration of mevalonic acid completely prevented the maternal and neonatal mortality but did not prevent low body weights in pups at 24 mg/kg on days 0 and 7 postpartum.
-
14 CLINICAL STUDIES
14.1 Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
In 12 placebo-controlled studies in patients with primary hypercholesterolemia and mixed dyslipidemia, fluvastatin capsules were administered to 1621 patients in daily dose regimens of 20 mg, 40 mg, and 80 mg (40 mg twice daily) for at least 6 weeks duration (Table 5). After 24 weeks of treatment, treatment with fluvastatin capsules resulted in significantly reduced plasma LDL-C, TC, TG, and Apo B compared to placebo and was associated with variable increases in HDL-C across the dose range.
In patients with primary mixed dyslipidemia as defined by baseline plasma TG levels ≥ 200 mg/dL and < 400 mg/dL, treatment with fluvastatin capsules produced significant decreases in Total-C, LDL-C, TG and Apo B and variable increases in HDL-C (Table 5).
Table 5: Median Percent Change in Lipid Parameters From Baseline to Week 24 Endpoint All Placebo-Controlled Studies (Fluvastatin Capsules) Total Chol
TG
LDL
Apo B
HDL
Dose
N
% Δ
N
% Δ
N
% Δ
N
% Δ
N
% Δ
All Patients
fluvastatin capsules 20 mg1
747
-17
747
-12
747
-22
114
-19
747
+3
fluvastatin capsules 40 mg1
748
-19
748
-14
748
-25
125
-18
748
+4
fluvastatin capsules 40 mg twice daily1
257
-27
257
-18
257
-36
232
-28
257
+6
Baseline TG ≥ 200 mg/dL
fluvastatin capsules 20 mg1
148
-16
148
-17
148
-22
23
-19
148
+6
fluvastatin capsules 40 mg1
179
-18
179
-20
179
-24
47
-18
179
+7
fluvastatin capsules 40 mg twice daily1
76
-27
76
-23
76
-35
69
-28
76
+9
1. Data for fluvastatin capsules from 12 placebo-controlled trials
14.2 Heterozygous Familial Hypercholesterolemia in Pediatric Patients
Fluvastatin capsules were studied in two open-label, uncontrolled, dose-titration studies. The first study enrolled 29 pre-pubertal boys, 9 to 12 years of age, who had an LDL-C level > 90th percentile for age and one parent with primary hypercholesterolemia and either a family history of premature ischemic heart disease or tendon xanthomas. The mean baseline LDL-C was 226 mg/dL (range: 137 to 354 mg/dL). All patients were started on fluvastatin capsules, 20 mg daily with dose adjustments every 6 weeks to 40 mg daily then 80 mg daily (40 mg b.i.d.) to achieve an LDL-C goal between 96.7 to 123.7 mg/dL. Endpoint analyses were performed at Year 2. Fluvastatin capsules decreased plasma levels of Total-C and LDL-C by 21% and 27%, respectively. The mean achieved LDL-C was 161 mg/dL (range: 74 to 336 mg/dL).
The second study enrolled 85 male and female patients, 10 to 16 years of age, who had an LDL-C > 190 mg/dL or LDL-C > 160 mg/dL and one or more risk factors for coronary heart disease, or LDL-C > 160 mg/dL and a proven LDL-receptor defect. The mean baseline LDL-C was 225 mg/dL (range: 148 to 343 mg/dL). All patients were started on fluvastatin capsules 20 mg daily with dose adjustments every 6 weeks to 40 mg daily then 80 mg daily (fluvastatin sodium extended-release tablets, 80 mg) to achieve an LDL-C goal of < 130 mg/dL. Endpoint analyses were performed at Week 114. Fluvastatin sodium decreased plasma levels of Total-C and LDL-C by 22% and 28%, respectively. The mean achieved LDL-C was 159 mg/dL (range: 90 to 295 mg/dL).
The majority of patients in both studies (83% in the first study and 89% in the second study) were titrated to the maximum daily dose of 80 mg. At study endpoint, 26% to 30% of patients in both studies achieved a targeted LDL-C goal of < 130 mg/dL. The long-term efficacy of fluvastatin sodium therapy in childhood to reduce morbidity and mortality in adulthood has not been established.
14.3 Secondary Prevention of Cardiovascular Disease
In the Fluvastatin Capsules Intervention Prevention Study, the effect of fluvastatin capsules, 40 mg administered twice daily on the risk of recurrent cardiac events (time to first occurrence of cardiac death, nonfatal myocardial infarction, or revascularization) was assessed in 1677 patients with CHD who had undergone a percutaneous coronary intervention (PCI) procedure (mean time from PCI to randomization = 3 days). In this multicenter, randomized, double-blind, placebo-controlled study, patients were treated with dietary/lifestyle counseling and either fluvastatin capsules, 40 mg (n = 844) or placebo (n = 833) given twice daily for a median of 3.9 years. The study population was 84% male, 98% Caucasian, with 37% > 65 years of age. Mean baseline lipid concentrations were: total cholesterol 201 mg/dL, LDL-C 132 mg/dL, triglycerides 70 mg/dL and HDL-C 39 mg/dL.
Fluvastatin capsules significantly reduced the risk of recurrent cardiac events (Figure 1) by 22% (p = 0.013, 181 patients in the fluvastatin capsules group vs. 222 patients in the placebo group). Revascularization procedures comprised the majority of the initial recurrent cardiac events (143 revascularization procedures in the fluvastatin capsules group and 171 in the placebo group). Consistent trends in risk reduction were observed in patients > 65 years of age.
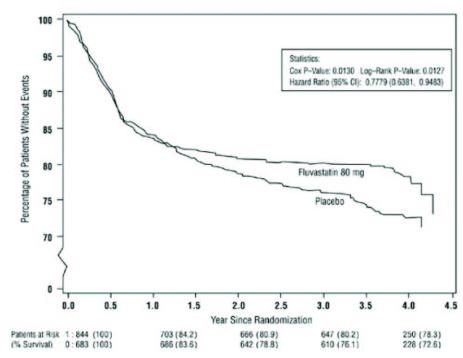
Figure 1: Primary Endpoint - Recurrent Cardiac Events (Cardiac Death, Nonfatal MI or Revascularization Procedure) (ITT Population)
Outcome data for the Fluvastatin Capsules Intervention Prevention Study are shown in Figure 2. After exclusion of revascularization procedures (CABG and repeat PCI) occurring within the first 6 months of the initial procedure involving the originally instrumental site, treatment with fluvastatin capsules was associated with a 32% (p = 0.002) reduction in risk of late revascularization procedures (CABG or PCI occurring at the original site > 6 months after the initial procedure, or at another site).
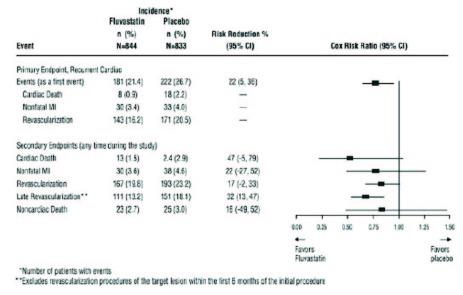
Figure 2: Fluvastatin Capsules Intervention Prevention Study - Primary and Secondary Endpoints
In the Lipoprotein and Coronary Atherosclerosis Study (LCAS), the effect of fluvastatin capsule therapy on coronary atherosclerosis was assessed by quantitative coronary angiography (QCA) in patients with CAD and mild to moderate hypercholesterolemia (baseline LDL-C range 115 to 190 mg/dL). In this randomized double-blind, placebo-controlled trial, 429 patients were treated with conventional measures (Step 1 AHA Diet) and either fluvastatin capsules, 40 mg/day or placebo. In order to provide treatment to patients receiving placebo with LDL-C levels ≥ 160 mg/dL at baseline, adjunctive therapy with cholestyramine was added after Week 12 to all patients in the study with baseline LDL-C values of ≥ 160 mg/dL which were present in 25% of the study population. Quantitative coronary angiograms were evaluated at baseline and 2.5 years in 340 (79%) angiographic evaluable patients.
Compared to placebo, fluvasatin capsules significantly slowed the progression of coronary atherosclerosis as measured by within-patient per-lesion change in minimum lumen diameter (MLD), the primary endpoint (Figure 3 below), percent diameter stenosis (Figure 4), and the formation of new lesions (13% of all fluvastatin patients versus 22% of all placebo patients). A significant difference in favor of fluvastatin capsules was found between all fluvastatin and all placebo patients in the distribution among the three categories of definite progression, definite regression, and mixed or no change. Beneficial angiographic results (change in MLD) were independent of patients’ gender and consistent across a range of baseline LDL-C levels.
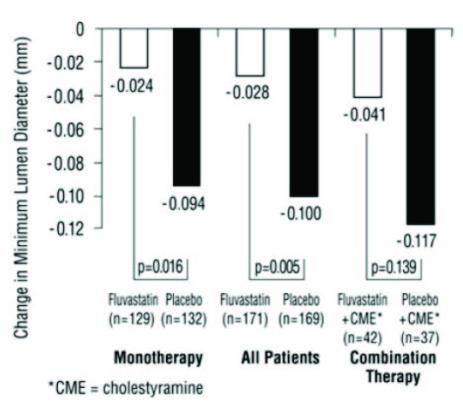
Figure 3: Change in Minimum Lumen Diameter (mm)
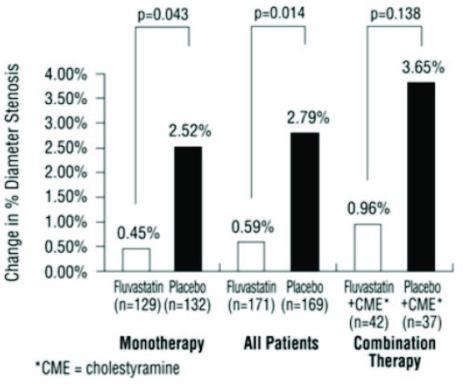
Figure 4: Change in % Diameter Stenosis
-
15 REFERENCES
- National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 89(3):495-501.1992.
- Manson, J.M., Freyssinges, C., Ducrocq, M.B., Stephenson, W.P., Postmarketing Surveillance of Lovastatin and Simvastatin Exposure During Pregnancy, Reproductive Toxicology, 10(6): 439-446, 1996.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Fluvastatin Capsules, USP are available as follows:
40 mg - Hard gelatin capsules with yellow opaque body and pink opaque cap, filled with an off-white to yellowish powder with small agglomerates, cap imprinted with “TEVA” and body imprinted with “7443”, in bottles 100 (NDC: 63629-8737-1).
Store and Dispense
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required). Protect from light.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
-
17 PATIENT COUNSELING INFORMATION
Information for Patients
Patients taking fluvastatin capsules should be advised that high cholesterol is a chronic condition and they should adhere to their medication along with their National Cholesterol Education Program (NCEP)-recommended diet, a regular exercise program, and periodic testing of a fasting lipid panel to determine goal attainment.
Patients should be advised about substances they should not take concomitantly with fluvastatin capsules [see Warnings and Precautions (5.1)]. Patients should also be advised to inform other healthcare professionals prescribing a new medication that they are taking fluvastatin capsules.
17.1 Muscle Pain
Patients starting therapy with fluvastatin capsules should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever or if these muscle signs or symptoms persist after discontinuing fluvastatin.
17.2 Liver Enzymes
It is recommended that liver enzyme tests be performed before the initiation of fluvastatin capsules and if signs or symptoms of liver injury occur. All patients treated with fluvastatin capsules should be advised to report promptly any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
17.3 Pregnancy
Women of childbearing age should be advised to use an effective method of birth control to prevent pregnancy while using fluvastatin capsules. Discuss future pregnancy plans with your patients, and discuss when to stop taking fluvastatin capsules if they are trying to conceive. Patients should be advised that if they become pregnant they should stop taking fluvastatin capsules and call their healthcare professional.
17.4 Breastfeeding
Women who are breastfeeding should not use fluvastatin capsules. Patients who have a lipid disorder and are breastfeeding should be advised to discuss the options with their healthcare professional.
Manufactured In Israel By:
Teva Pharmaceutical Ind. Ltd.
Kfar Saba, 4410202, Israel
Manufactured For:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
Rev. I 8/2020
-
PATIENT PACKAGE INSERT
FDA-Approved Patient Labeling
Fluvastatin (floo'' va stat' in) Capsules
20 mg, 40 mg
You must read and follow all instructions before using fluvastatin capsules.
Read the Patient Information every time you or a family member gets fluvastatin capsules. There may be new information. This Patient Information does not take the place of talking with your doctor about your medical condition or treatment. If you have any questions about fluvastatin capsules, ask your doctor or pharmacist.
What are fluvastatin capsules?
Fluvastatin capsules are a prescription medicine called "statins" that lower cholesterol in your blood. They lower the "bad" cholesterol and triglycerides in your blood. They can raise your "good" cholesterol as well.
Fluvastatin capsules are for people whose cholesterol does not come down enough with exercise and a low-fat diet alone.
Fluvastatin capsules may be used in patients with heart disease (coronary artery disease) to:
- lower the chances of heart problems which would require procedures to help restore blood flow to the heart.
- slow the buildup of too much cholesterol in the arteries of the heart.
Treatment with fluvastatin capsules has not been shown to prevent heart attacks or stroke.
Fluvastatin capsules are taken one or two times a day.
Who should not take fluvastatin capsules?
Do not take fluvastatin capsules if you:
- are pregnant or think you may be pregnant, or are planning to become pregnant. Fluvastatin capsules may harm your unborn baby. If you get pregnant, stop taking fluvastatin capsules and call your doctor right away.
- are breast-feeding. Fluvastatin sodium can pass into your breast milk and may harm your baby
- have liver problems
- are allergic to fluvastatin capsules or any of their ingredients. The active ingredient in fluvastatin capsules is fluvastatin. See the end of this leaflet for a complete list of ingredients in fluvastatin capsules.
Fluvastatin capsules have not been studied in children under 9 years of age.
Before taking fluvastatin capsules, tell your doctor if you:
- have muscle aches or weakness
- drink more than 2 glasses of alcohol daily
- have diabetes
- have a thyroid problem
- have kidney problems
Some medicines should not be taken with fluvastatin capsules. Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Fluvastatin capsules and certain other medicines can interact causing serious side effects. Especially tell your doctor if you take medicines for:
- your immune system
- cholesterol
- infections
- heart failure
- seizures
- diabetes
- heartburn or stomach ulcers
Know all the medicines you take. Keep a list of all the medicines you take with you to show your doctor and pharmacist.
How should I take fluvastatin capsules?
- Your doctor will prescribe the medicine that is right for you. Take fluvastatin capsules exactly as prescribed. Do not change your dose or stop fluvastatin capsules without talking to your doctor. Your doctor may do blood tests to check your cholesterol levels during treatment with fluvastatin capsules. Your dose of fluvastatin capsules may be changed based on these blood test results.
- Take fluvastatin capsules at the same time every evening. When fluvastatin capsules are taken twice daily, the capsules may be taken once in the morning and once in the evening. Fluvastatin capsules can be taken with or without food.
- Do not open fluvastatin capsules.
- Your doctor should start you on a low-fat and low-cholesterol diet before giving you fluvastatin capsules. Stay on this low-fat and low-cholesterol diet while taking fluvastatin capsules.
- If you miss a dose of fluvastatin capsules, take it as soon as you remember. Do not take fluvastatin capsules if it has been more than 12 hours since your last dose. Wait and take the next dose at your regular time. Do not take 2 doses of fluvastatin capsules at the same time.
- If you take too many fluvastatin capsules or overdose, call your doctor or Poison Control Center right away. Or, go to the nearest emergency room.
What should I avoid while taking fluvastatin capsules?
- Talk to your doctor before you start any new medicines. This includes prescription and nonprescription medicines, vitamins and herbal supplements. Fluvastatin capsules and certain other medicines can interact causing serious side effects.
- Do not get pregnant. If you get pregnant, stop taking fluvastatin capsules right away and call your doctor.
What are the possible side effects of fluvastatin capsules?
When taking fluvastatin capsules, some patients may develop serious side effects, including:
muscle problems. Call your health care professional right away if you experience unexplained muscle pain, tenderness, or weakness especially with fever. This may be an early sign of a rare muscle problem that could lead to serious kidney problems.
The risk of muscle problems is greater in people who are 65 years of age or older, or who already have thyroid or kidney problems. The chance of muscle problems may be increased if you are taking certain other medicines with fluvastatin capsules.
If you have muscle problems that do not go away even after your health care professional has advised you to stop taking fluvastatin capsules, notify your health care professional. Your health care professional may do further tests to diagnose the cause of your muscle problems.
liver problems. Your doctor should do blood tests to check your liver before you start taking fluvastatin capsules, and if you have symptoms of liver problems while you take fluvastatin capsules. Call your doctor right away if you have the following symptoms of liver problems:
- feel tired or weak
- loss of appetite
- upper belly pain
- dark amber colored urine
- yellowing of your skin or the whites of your eyes
The most common side effects of fluvastatin capsules are headache, upset stomach and stomach pain, diarrhea, flu-like symptoms, muscle pain, sinus infection, tiredness, or trouble sleeping. These side effects are usually mild and may go away. The following additional side effects have been reported with fluvastatin capsules: memory loss, and confusion.
Talk to your doctor or pharmacist if you have side effects that bother you or that will not go away.
These are not all the side effects of fluvastatin capsules. Ask your doctor or pharmacist for a complete list.
How should I store fluvastatin capsules?
- Store fluvastatin capsules at room temperature, 20° to 25°C (68° to 77°F). Protect from light.
- Do not keep medicine that is out of date or that you no longer need.
- Keep fluvastatin capsules out of the reach of children. Be sure that if you throw medicines away, they are out of the reach of children.
General information about fluvastatin capsules
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use fluvastatin capsules for a condition for which they were not prescribed. Do not give fluvastatin capsules to other people, even if they have the same problem you have; they may harm them.
For more information, call 1-888-838-2872.
What are the ingredients in fluvastatin capsules?
Active Ingredient: fluvastatin sodium (hydrated form)
Inactive Ingredients: black iron oxide, colloidal silicon dioxide, crospovidone, gelatin, lactose monohydrate, magnesium stearate, propylene glycol, red iron oxide, shellac, titanium dioxide, and yellow iron oxide. The imprinting ink may contain potassium hydroxide.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Manufactured In Israel By:
Teva Pharmaceutical Ind. Ltd.
Kfar Saba, 4410202, Israel
Manufactured For:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
Rev. E 7/2020
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLUVASTATIN
fluvastatin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63629-8737(NDC:0093-7443) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUVASTATIN SODIUM (UNII: PYF7O1FV7F) (FLUVASTATIN - UNII:4L066368AS) FLUVASTATIN 40 mg Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERRIC OXIDE RED (UNII: 1K09F3G675) SHELLAC (UNII: 46N107B71O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color yellow, pink Score no score Shape CAPSULE Size 19mm Flavor Imprint Code TEVA;7443 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-8737-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/19/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078407 07/05/2012 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-8737) , RELABEL(63629-8737)