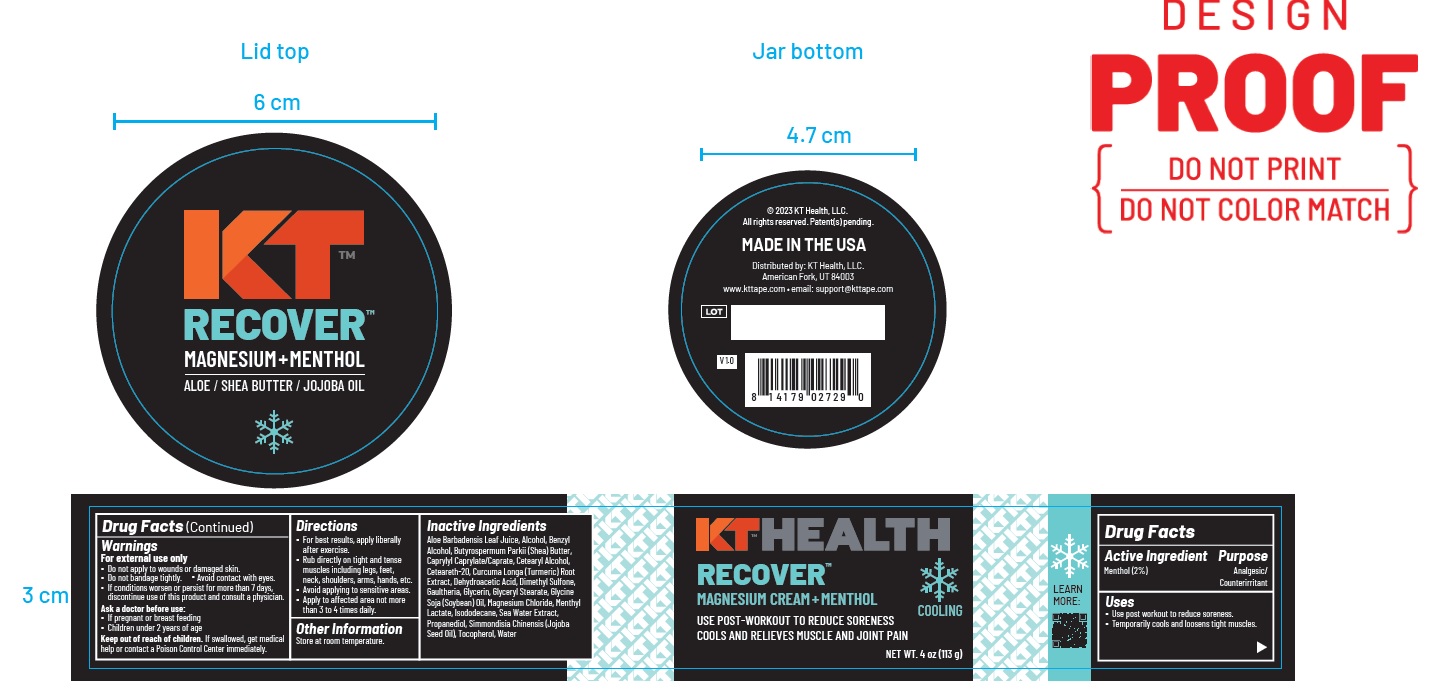
Label: KT RECOVER MAGNESIUM- menthol cream
- NDC Code(s): 73044-104-01
- Packager: KT Health LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
Warnings
For external use only
- Do not apply to wounds or damaged skin.
- Do not bandage tightly.
- Avoid contact with eyes.
- If condition worsens, or persist for more than 7 days, discontinue use of this product and consult a physician.
- Ask a doctor before use:
- If pregnant or breast feeding
- Children under 2 years of age
Keep out of reach of children.If swallowed, get medical
help or contact a Poison Control Center immediately. - KEEP OUT OF REACH OF CHILDREN
- Directions
-
INACTIVE INGREDIENT
Aloe Barbadensis Leaf Juice, Alcohol, Benzyl Alcohol, Butyrospermum Parkii (Shea) Butter, Caprylyl Caprylate/Caprate, Cetearyl Alcohol,
Ceteareth-20, Curcuma Longa (Turmeric) Root Extract, Dehydroacetic Acid, Dimethyl Sulfone,Gaultheria, Glycerin, Glyceryl Stearate, Glycine
Soja (Soybean) Oil, Magnesium Chloride, Menthyl Lactate, Isododecane, Sea Water Extract, Propanediol, Simmondisia Chinensis (Jojoba Seed Oil), Tocopherol, Water - Product label
-
INGREDIENTS AND APPEARANCE
KT RECOVER MAGNESIUM
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73044-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ALCOHOL (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) SHEA BUTTER (UNII: K49155WL9Y) CAPRYLYL CAPRYLATE/CAPRATE (UNII: 22MCG4RSMR) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) TURMERIC (UNII: 856YO1Z64F) DEHYDROACETIC ACID (UNII: 2KAG279R6R) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) METHYL SALICYLATE (UNII: LAV5U5022Y) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCINE (UNII: TE7660XO1C) SOYBEAN OIL (UNII: 241ATL177A) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ISODODECANE (UNII: A8289P68Y2) SODIUM CHLORIDE (UNII: 451W47IQ8X) PROPANEDIOL (UNII: 5965N8W85T) JOJOBA OIL (UNII: 724GKU717M) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73044-104-01 4 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/12/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/12/2023 Labeler - KT Health LLC (807008037)